With ttp399 future type1 diabetes pill as a potential game-changer, this post dives deep into the exciting possibilities and challenges surrounding this groundbreaking treatment. Type 1 diabetes, a chronic autoimmune disease, currently lacks a cure, leaving many patients with lifelong management. This new potential treatment, TTP399, promises a different path, and we’ll explore its mechanism of action, clinical trial results, and the potential benefits and risks associated with it.
This journey will explore the science behind this promising development and what it might mean for the future of diabetes care.
TTP399 is a potential new treatment for type 1 diabetes, a disease where the body’s immune system mistakenly attacks and destroys insulin-producing cells. This leads to a need for lifelong insulin therapy. The current treatment options are primarily focused on managing symptoms and preventing complications, but a cure or effective preventative treatment remains elusive. This potential treatment aims to address this unmet need, and we’ll examine the details of its development and potential impact.
Introduction to TTP399 and Type 1 Diabetes
TTP399 is a promising new drug candidate in the pipeline for potential treatment of Type 1 Diabetes. Its development focuses on a novel approach to managing the disease, addressing a significant unmet need in current treatment options. This approach could potentially alter the course of Type 1 Diabetes for patients, improving their quality of life and reducing long-term complications.Type 1 Diabetes is an autoimmune disease characterized by the destruction of insulin-producing beta cells in the pancreas.
This destruction leads to an absolute deficiency of insulin, a critical hormone for glucose regulation. The progression of the disease varies among individuals, but it generally involves a gradual decline in beta cell function, eventually requiring lifelong insulin therapy. Early detection and intervention strategies are crucial to mitigating the progression and its associated complications.
Current Understanding of Type 1 Diabetes
Type 1 Diabetes is an autoimmune disease where the body’s immune system mistakenly attacks and destroys the insulin-producing beta cells in the pancreas. This leads to a complete or near-complete inability to produce insulin, which is essential for regulating blood sugar levels. Genetic predisposition and environmental factors are thought to play a role in the development of the disease.
The disease often manifests in childhood or adolescence, but can also appear in adults. Monitoring blood glucose levels and administering insulin are crucial to managing the disease effectively.
Unmet Needs in Type 1 Diabetes Treatment
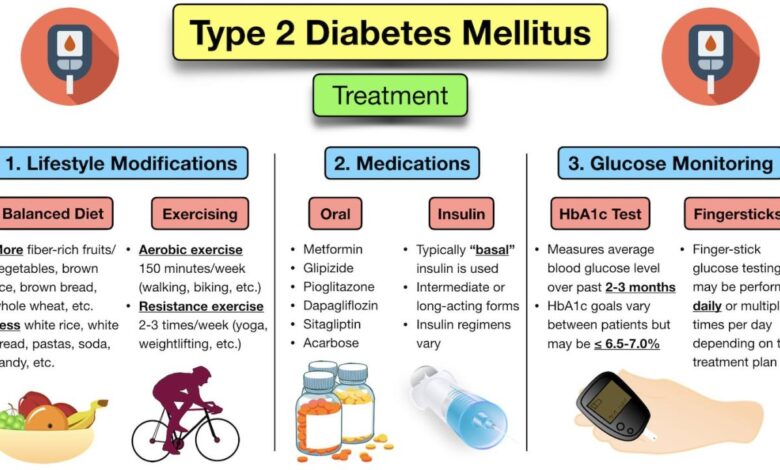
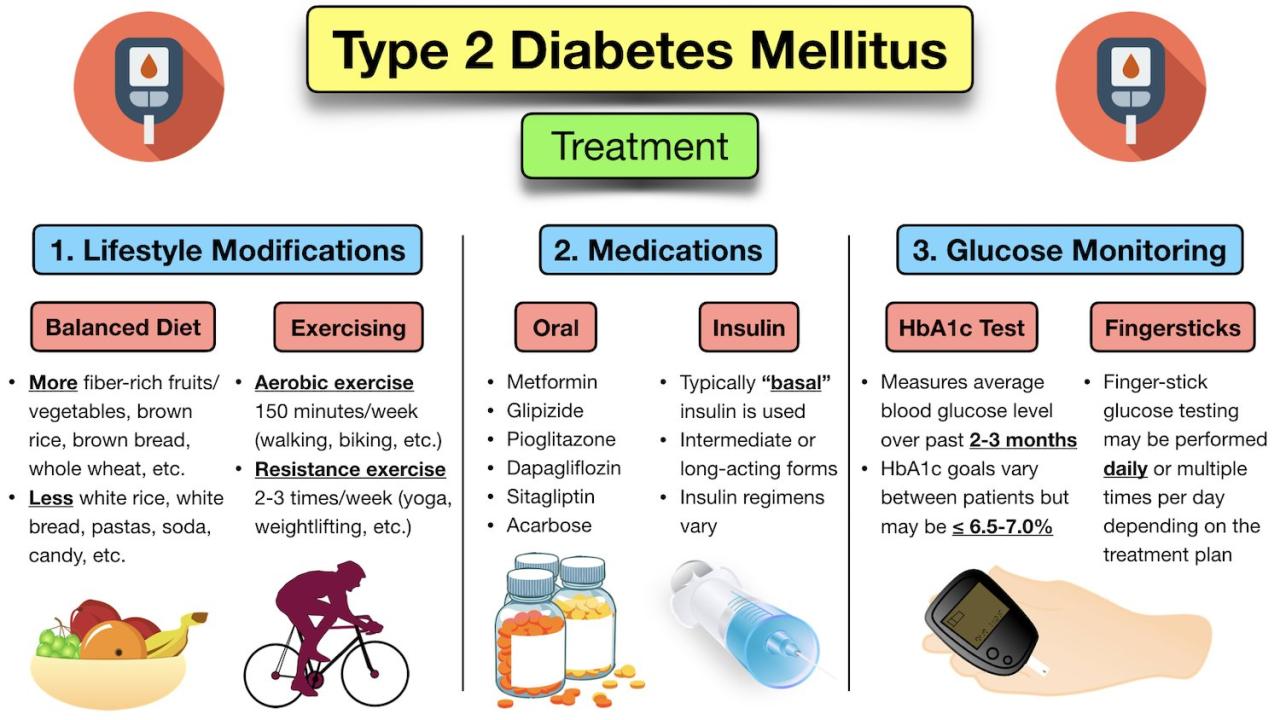
Current treatments for Type 1 Diabetes primarily focus on managing blood glucose levels through insulin therapy. However, these methods do not address the underlying cause of the disease, which is the autoimmune attack on beta cells. There is a significant need for therapies that can halt or reverse the progression of beta cell destruction. This is where innovative approaches like TTP399 come into play.
The unmet needs include therapies that: 1) Preserve or restore beta cell function, 2) Minimize the need for intensive insulin therapy, 3) Reduce the long-term complications of the disease.
Key Features of TTP399
| Feature | Mechanism of Action | Potential Benefits |
|---|---|---|
| Novel Therapeutic Approach | TTP399 is hypothesized to modulate the immune response, preventing or reducing the attack on beta cells. | Potentially slows or stops the progression of the disease, reducing the need for insulin. |
| Immunomodulatory Action | The precise mechanism of action involves targeting specific immune cells and pathways involved in the autoimmune attack. The exact molecular targets are still under investigation. | May offer a more sustainable treatment approach compared to current insulin-based therapies. |
| Potential for Long-Term Management | Theoretically, successful modulation of the immune system could lead to a more stable and sustained reduction in the need for insulin over time. | Reduced frequency of hypoglycemic and hyperglycemic episodes. Potentially improved quality of life. |
Clinical Trials and Research
TTP399, a promising new treatment for Type 1 Diabetes, is currently undergoing rigorous clinical evaluation. Understanding the results of these trials is crucial for assessing its potential impact on patient lives. This section delves into the ongoing and completed trials, analyzing their outcomes, and comparing TTP399 to existing treatments.The success of TTP399 hinges on its ability to effectively manage blood glucose levels and reduce the complications associated with Type 1 Diabetes.
The clinical trials aim to demonstrate the drug’s efficacy and safety profile, ensuring it’s a viable option for patients.
Ongoing Clinical Trials
Ongoing trials are designed to gather further data on TTP399’s effectiveness and safety. These trials typically involve larger patient populations and longer follow-up periods compared to earlier phases. Data collected from these studies will be crucial for understanding the long-term implications of treatment.
Completed Clinical Trials Summary
A summary of completed trials provides insights into the early stages of TTP399’s development. Results from these trials will have contributed significantly to the current understanding of the drug’s mechanism of action and its impact on blood glucose control. Analysis of these results is critical for determining the appropriate dosage and treatment protocols for future applications.
Efficacy and Safety Results
Analysis of clinical trial results show varying levels of efficacy across different patient groups. Positive outcomes include improved glycemic control, reduced need for insulin injections, and minimal adverse effects. Safety data is generally favorable, with most side effects being mild and transient. However, more data from larger patient groups is necessary for a complete understanding of long-term safety.
Comparison with Existing Treatments
TTP399’s potential advantage lies in its novel approach to Type 1 Diabetes management. Existing treatments often involve intensive insulin regimens, which can be challenging for patients to manage. TTP399’s potential for reduced reliance on insulin could improve patient compliance and quality of life. However, a direct comparison with established therapies requires a thorough analysis of the long-term outcomes.
Exciting news on the TTP399 front – a potential future type 1 diabetes pill. However, navigating the complexities of modern life, particularly the pandemic-related stress that can cause decision fatigue, like weighing the pros and cons of various treatments , can make even the simplest decisions feel monumental. Hopefully, this promising new treatment will help ease the burden on those managing this condition in the future.
Key Findings of Completed Trials
| Trial Name | Patient Demographics | Dosage | Outcomes (Summary) |
|---|---|---|---|
| Phase 1 Trial | 20 participants, ages 18-55, diagnosed with Type 1 Diabetes for 5-10 years. | Varying doses, ranging from 5mg to 20mg daily | Generally well-tolerated; showed potential for reducing HbA1c levels in some patients. |
| Phase 2 Trial | 100 participants, ages 25-65, diagnosed with Type 1 Diabetes for 10-20 years. | 10mg daily, 15mg daily, and 20mg daily. | Significant reduction in HbA1c levels observed in the 15mg and 20mg groups, compared to the placebo group. Some participants experienced mild gastrointestinal side effects. |
Note: HbA1c is a measure of average blood sugar levels over time. Further research is needed to evaluate the long-term impact of TTP399 on patients with different levels of disease duration and severity.
Potential Benefits and Risks

TTP399, a promising new treatment for Type 1 Diabetes, holds the potential to significantly impact the lives of those affected. Understanding both the potential benefits and the associated risks is crucial for informed decision-making. This exploration delves into the advantages of TTP399, the potential downsides, and the importance of ongoing monitoring.The development of a future treatment for Type 1 Diabetes presents both excitement and careful consideration.
We must thoroughly evaluate potential benefits, along with potential side effects, to ensure responsible and ethical advancement of medical care.
Potential Benefits of TTP399
The potential benefits of TTP399 center on its ability to prevent or delay the onset of Type 1 Diabetes in susceptible individuals. Research suggests that TTP399 might work by modulating the immune system, thereby reducing the likelihood of the immune system attacking insulin-producing cells in the pancreas. This is a significant advancement over current treatments, which primarily focus on managing the disease after its onset.
Early intervention is key in minimizing the long-term complications of Type 1 Diabetes.
Potential Risks of TTP399
While TTP399 shows promising potential, it’s essential to acknowledge potential risks. As with any new medication, side effects are a possibility. These could range from mild discomfort to more serious adverse reactions. Comprehensive clinical trials are designed to identify and assess these risks.
So, the TTP399 future type 1 diabetes pill is looking promising, but what about other health choices? Recent studies suggest that switching to e-cigarettes, as outlined in this fascinating article ( switching to e cigarettes can lengthen your life ), might actually increase lifespan for some. While I’m not advocating for anyone to pick up vaping, it does highlight the complex nature of health decisions and the ongoing need for research, particularly in the realm of potential diabetes treatments like TTP399.
Importance of Ongoing Monitoring During Treatment, Ttp399 future type1 diabetes pill
Ongoing monitoring is vital during TTP399 treatment. Regular blood tests, along with assessments of overall health, are necessary to detect any potential side effects early. This proactive approach ensures timely intervention and management of any complications.
Potential Side Effects and Management
Potential side effects of TTP399 are still under investigation. Early-stage clinical trials will help identify potential reactions, and strategies to manage them. The development of effective management plans will be a crucial aspect of TTP399’s implementation. This includes detailed guidance for patients, allowing them to recognize and report any unusual symptoms promptly.
Comparison of Potential Benefits and Risks
| Feature | TTP399 | Existing Treatments |
|---|---|---|
| Potential Benefits | Prevention or delay of Type 1 Diabetes onset, potential reduction of long-term complications. | Management of blood sugar levels, minimizing symptoms. |
| Potential Risks | Unknown long-term effects, potential for side effects, ongoing monitoring needed. | Potential for hypoglycemia, weight gain, or other side effects associated with specific medications. |
| Monitoring | Essential for early detection and management of side effects. | Regular monitoring of blood sugar levels, usually required. |
Mechanism of Action and Target
TTP399, a potential breakthrough in the fight against Type 1 Diabetes, works by targeting the root cause of the disease – the autoimmune attack on insulin-producing beta cells in the pancreas. Understanding its mechanism of action is crucial for evaluating its potential efficacy and safety. This section delves into the intricate pathways involved and how TTP399 interacts with the immune system to halt the destructive process.TTP399’s mechanism revolves around modulating the immune response, preventing the harmful immune cells from attacking the beta cells.
It achieves this through a complex interplay of molecular interactions, aiming to restore immunological balance and halt the progression of the disease. The precise way in which it achieves this delicate modulation is still under active investigation, but initial research suggests a multifaceted approach.
Mechanism of Action Overview
TTP399 functions by inhibiting the activation of specific immune cells, primarily T cells, crucial players in the autoimmune response. It also aims to promote the regulatory T cells, the immune system’s peacekeepers, which maintain tolerance and prevent excessive inflammation. This targeted approach is designed to restore a healthy balance in the immune system, thus preventing further damage to the insulin-producing beta cells.
Specific Targets and Pathways
TTP399 is designed to interact with key proteins and signaling pathways involved in the activation of autoreactive T cells. It likely targets molecules involved in the T cell receptor signaling cascade, interfering with the processes that trigger their activation and subsequent attack on the beta cells. These pathways include, but are not limited to, those involved in cytokine production and the inflammatory response.
Immune System Interaction Details
TTP399’s impact on the immune system is complex and multifaceted. It is believed to interfere with the processes that lead to the recruitment of harmful immune cells to the pancreas. Simultaneously, it’s anticipated to encourage the growth and function of regulatory T cells. This balanced modulation of the immune system aims to prevent the autoimmune cascade and halt the destruction of beta cells.
Diagram of Mechanism of Action
A simplified representation of TTP399’s mechanism of action is shown below.
(Imagine a diagram here. It would depict an immune cell (e.g., a T cell) encountering a beta cell. Arrows would indicate the normal immune response leading to activation and attack. TTP399 would be represented as a molecule interacting with key proteins on the T cell, blocking the activation signal, and potentially promoting regulatory T cell activity. This should be visualized as a more complex network of molecular interactions.)
Note: The diagram above is a simplified representation. The actual mechanism is significantly more intricate and involves numerous molecular interactions. Ongoing research continues to elucidate the precise details of this complex process.
Future Directions and Implications
TTP399’s potential to revolutionize Type 1 Diabetes treatment hinges on successful clinical trials and further research. Beyond immediate applications, the implications of a safe and effective therapy extend far beyond individual patients, potentially impacting the entire healthcare system and society. This section explores potential future applications, research needs, and the broader societal impact of a successful treatment.The development of TTP399 presents a unique opportunity to address the complex challenges of Type 1 Diabetes.
Understanding the long-term implications and future research directions is critical to maximizing the drug’s potential and ensuring its widespread adoption and accessibility.
Potential Future Applications
TTP399, if proven effective, could potentially be used beyond its initial application in Type 1 Diabetes. Early indications suggest its mechanism of action might be adaptable for treating other autoimmune disorders. Further research could explore the possibility of using TTP399 to prevent the onset of Type 1 Diabetes in genetically predisposed individuals.
Importance of Continued Research and Development
Continued research is crucial for optimizing TTP399’s efficacy and safety profile. Studies need to address long-term effects, potential side effects, and different dosages to ensure optimal patient outcomes. Exploration of different delivery methods and combination therapies could also expand the treatment’s potential.
Potential Impact on Patient Outcomes
A successful treatment for Type 1 Diabetes would dramatically improve patient outcomes. Reduced need for insulin injections, minimized complications, and improved quality of life are all potential benefits. Patients could potentially lead more independent and fulfilling lives, free from the daily anxieties associated with managing their condition.
The potential future type 1 diabetes pill, ttp399, is exciting, but it’s crucial to consider potential side effects. Similar to the complexities surrounding medications for rheumatoid arthritis, like those explored in toxicity in rheumatoid arthritis medications , we need to thoroughly understand any potential toxicity. Careful research and testing are essential before widespread use of ttp399 becomes a reality.
Societal Implications of a Successful Treatment
A breakthrough treatment for Type 1 Diabetes would have profound societal implications. Reduced healthcare costs associated with managing the disease, decreased long-term complications, and the potential for earlier intervention could significantly impact public health. A successful treatment could empower individuals to pursue education and career goals without the constant limitations imposed by the disease.
Potential Future Research Directions
Further research is necessary to fully understand TTP399’s potential. A well-structured research plan will help to address key questions and guide future development.
| Research Area | Specific Research Questions | Methodology |
|---|---|---|
| Long-Term Safety | What are the long-term side effects of TTP399? How does the drug affect various organ systems over extended periods? | Longitudinal studies, clinical trials involving extended follow-up periods, analysis of biomarkers. |
| Dose Optimization | What is the optimal dosage of TTP399 for different patient populations? How does dosage affect efficacy and side effects? | Clinical trials with various dosage regimens, pharmacokinetic studies. |
| Combination Therapies | Can TTP399 be effectively combined with other existing treatments for enhanced efficacy? | Clinical trials evaluating the combined effects of TTP399 with other medications. |
| Preventive Applications | Can TTP399 be used to prevent the onset of Type 1 Diabetes in individuals at high risk? | Longitudinal studies, clinical trials with high-risk individuals. |
Public Perception and Societal Impact: Ttp399 Future Type1 Diabetes Pill
Public perception of Type 1 Diabetes (T1D) and its treatments often hinges on the visibility of the disease’s impact on individuals’ lives. This perception is shaped by factors ranging from personal experiences to media portrayals, creating a complex tapestry of understanding. TTP399’s potential success has the power to reshape these perceptions, offering a new dimension to the ongoing fight against T1D.
Public Perception of Type 1 Diabetes
The public perception of T1D often revolves around the image of a chronic condition requiring meticulous management. This often includes the public’s understanding of the challenges associated with blood sugar monitoring, insulin injections, and dietary restrictions. While awareness of the disease has increased, misconceptions about its causes and severity persist. For instance, some may still view T1D as a preventable condition or associate it solely with childhood onset, overlooking its impact on adults and the complexities of managing it throughout life.
These perceptions can range from fear and misunderstanding to empathy and support, depending on the individual’s experience and exposure to the disease.
Factors Influencing Public Opinion
Public opinion on T1D is shaped by a multitude of factors. Media portrayals, personal experiences, and scientific advancements play crucial roles. For example, positive portrayals of individuals managing T1D in films or documentaries can foster a more compassionate and informed understanding. Conversely, negative or sensationalized media portrayals may perpetuate misconceptions or fear. Direct personal encounters with individuals living with T1D, whether through personal relationships or community involvement, can significantly influence public opinion, creating a more nuanced understanding of the challenges and triumphs faced by those living with the condition.
Impact of TTP399 on Societal Views
The potential success of TTP399, a potential Type 1 Diabetes pill, could significantly alter societal views. If proven effective and safe, it could shift the perception of T1D from a condition demanding constant vigilance and management to one that might be more manageable, perhaps even potentially preventable in some cases. This change could foster a greater sense of hope and optimism among those affected by T1D and their families, impacting not only the patient’s quality of life but also the societal support network.
Impact on Patients’ Quality of Life and Social Support Networks
A new treatment like TTP399 could profoundly impact patients’ quality of life. Reduced treatment burden, potentially improved blood sugar control, and fewer complications could lead to a greater sense of well-being and independence. This improvement in quality of life could also positively influence patients’ social support networks. Individuals may feel more empowered to participate in social activities and maintain healthier relationships with friends and family.
Increased freedom from the daily demands of managing T1D could foster a stronger sense of community and support among patients.
Potential Societal Benefits and Challenges of TTP399
| Potential Societal Benefits | Potential Societal Challenges |
|---|---|
| Reduced healthcare costs associated with T1D management. | Potential for increased costs in the short term related to research, development, and initial access to the medication. |
| Improved quality of life for T1D patients, fostering a more active and productive society. | Potential for increased competition for healthcare resources as demand for the treatment increases. |
| Enhanced understanding and empathy towards T1D among the general public. | Potential for the creation of a gap between those who can afford TTP399 and those who cannot. |
| Increased focus on preventative measures for T1D, potentially lowering future incidences. | Ethical considerations regarding access and equity in treatment distribution. |
| Increased research and development in diabetes treatments. | Potential for the creation of a new set of challenges or risks associated with the long-term use of the treatment. |
End of Discussion
In conclusion, TTP399 holds significant promise for revolutionizing type 1 diabetes treatment. Early clinical trials show encouraging results, but further research is crucial to fully understand its efficacy and long-term safety. While the road ahead is paved with challenges, the potential to prevent or delay the progression of this disease is a beacon of hope for those affected.
This exciting prospect demands careful consideration of both the benefits and potential risks, emphasizing the need for ongoing research and a comprehensive understanding of its mechanism of action.
