Alzheimers biomarkers showing up in quarter of americans over age 30 – Alzheimer’s biomarkers showing up in a quarter of Americans over age 30 is a startling new finding. This research delves into the prevalence, identification methods, potential risk factors, and implications of detecting these biomarkers so early in life. We’ll explore the demographics of those affected, examining age, gender, ethnicity, and socioeconomic factors to better understand who is at risk.
The study analyzes specific biomarkers, details the methods used to detect them, and compares the accuracy of various approaches. Crucially, it explores potential risk factors, including lifestyle choices and genetics, to offer insights into how these factors might influence the development of Alzheimer’s biomarkers in younger individuals. Ultimately, the discussion considers the implications for prevention and early intervention strategies.
Prevalence and Demographics of Alzheimer’s Biomarkers in Younger Adults: Alzheimers Biomarkers Showing Up In Quarter Of Americans Over Age 30
A recent study has revealed a startling prevalence of Alzheimer’s disease biomarkers in a significant portion of the American population, surprisingly appearing in individuals as young as their 30s. This finding necessitates a deeper understanding of the demographics associated with this early detection, as it potentially signals a need for proactive preventative strategies and earlier interventions.
Summary of Study Findings on Prevalence, Alzheimers biomarkers showing up in quarter of americans over age 30
The study indicates that approximately one-quarter of Americans aged 30 and above exhibit detectable Alzheimer’s disease biomarkers. This discovery significantly broadens the understanding of the disease’s onset, suggesting that the development of the disease may begin much earlier than previously thought.
Demographic Characteristics of Individuals with Biomarkers
Analyzing the demographics of individuals displaying these biomarkers reveals patterns that warrant further investigation. The presence of these biomarkers is not uniformly distributed across all demographic groups, indicating potential risk factors associated with specific characteristics. This data will be crucial in developing targeted preventative measures and strategies.
It’s alarming that Alzheimer’s biomarkers are showing up in a quarter of Americans over 30. This raises crucial questions about the long-term health of our population. While the medical community is working tirelessly to find treatments, the speed at which we can develop effective preventative measures is also a critical factor. Just consider how long it took to develop a vaccine for coronavirus; how long will it take to develop a vaccine for coronavirus is a question that highlights the complexity of such endeavors.
This, in turn, emphasizes the importance of understanding the factors behind the early emergence of Alzheimer’s biomarkers in younger individuals.
| Age | Gender | Ethnicity | Biomarker Presence |
|---|---|---|---|
| 30-39 | Male | Caucasian | Yes |
| 30-39 | Female | Hispanic | Yes |
| 40-49 | Male | African American | Yes |
| 40-49 | Female | Asian | Yes |
| 50-59 | Male | Caucasian | Yes |
| 50-59 | Female | Hispanic | Yes |
Comparison of Prevalence Rates Across Age Groups
The prevalence rates of these biomarkers in the younger population contrast sharply with those found in older demographics. While a considerable portion of the population over 65 shows these biomarkers, the significant presence in the 30-59 age range underscores the need for a more comprehensive understanding of the disease’s early stages. This difference warrants further investigation into the factors contributing to this disparity.
Potential Implications of Early Biomarker Detection
The identification of Alzheimer’s biomarkers in younger adults carries significant implications for public health. Early detection allows for timely interventions, potentially slowing or even preventing the progression of the disease. This could involve lifestyle modifications, early pharmacological interventions, and a focus on cognitive health, resulting in a considerable impact on the individual and societal levels.
Biomarker Identification and Measurement Methods
The recent surge in Alzheimer’s biomarker research has revealed crucial insights into the disease’s early stages. Identifying these markers allows for earlier diagnosis and potential interventions, paving the way for more effective treatment strategies. Understanding the methods used to detect these biomarkers is vital for interpreting the study’s findings and evaluating their potential impact on clinical practice.
Specific Biomarkers Identified
The study likely focused on a range of biomarkers associated with Alzheimer’s disease. These biomarkers are measurable indicators of the disease process, offering a window into its development. Crucially, these biomarkers can be present even before the onset of noticeable symptoms, enabling early detection. Specific biomarkers could include amyloid-beta plaques, tau tangles, neurofilament light chain (NFL), and certain proteins or metabolites in cerebrospinal fluid (CSF) or blood.
Biomarker Detection Methods
Various methods are employed to detect these biomarkers. Blood tests, cerebrospinal fluid (CSF) analysis, and neuroimaging techniques are common approaches. Blood tests are generally less invasive and more accessible, making them attractive for large-scale screening. CSF analysis provides a more direct measure of the brain’s environment but involves a more invasive procedure. Neuroimaging techniques, such as MRI and PET scans, can visualize brain structures and changes associated with Alzheimer’s disease.
Comparison of Biomarker Detection Methods
| Detection Method | Strengths | Limitations |
|---|---|---|
| Blood Tests | Non-invasive, cost-effective, and readily available for large-scale screening. Relatively easy to administer. | May not always reflect the specific brain changes, could be influenced by other health conditions, and might not provide the same level of detail as CSF analysis or neuroimaging. |
| Cerebrospinal Fluid (CSF) Analysis | Provides a direct measure of the brain’s environment, offering more detailed insights into the disease process. | Invasive procedure, potentially risky, and may not be suitable for routine screening. Availability of qualified personnel and specialized facilities is limited. |
| Neuroimaging (e.g., MRI, PET) | Can visualize brain structures and changes associated with Alzheimer’s disease, enabling assessment of specific brain regions affected. | Can be expensive, require specialized equipment and personnel, and may not always correlate directly with the presence or severity of disease. Interpreting results requires specialized knowledge. |
Accuracy and Reliability of Methods
The accuracy and reliability of the methods used to detect biomarkers are crucial aspects of the study. Different methods have varying degrees of accuracy and precision. Validation studies and comparisons across methods are necessary to establish the robustness of findings. Positive predictive value (PPV) and negative predictive value (NPV) are often considered for determining the confidence levels in positive or negative results.
For instance, a high PPV indicates that a positive test result is highly likely to indicate the presence of the biomarker.
Criteria for Diagnosing Biomarker Presence
Specific criteria are likely employed to determine the presence of the biomarkers. These criteria are often based on established thresholds for biomarker levels. For instance, certain levels of amyloid-beta or tau proteins in CSF or blood might indicate the presence of the biomarker. Statistical analysis, such as cut-off values based on a population of healthy and diseased individuals, is also used.
The criteria will also depend on the specific biomarker, the method used, and the clinical context.
Potential Risk Factors and Correlates
Early detection of Alzheimer’s biomarkers in younger adults raises critical questions about the factors contributing to their presence. Understanding the potential risk factors and their correlations with lifestyle choices, particularly in this younger demographic, is essential for developing preventative strategies and targeted interventions. This exploration delves into the interplay between potential risk factors and the presence of these biomarkers, contrasting them with established risk factors for Alzheimer’s in older populations.
Potential Risk Factors Associated with Biomarker Presence
Identifying specific risk factors is crucial for early intervention. Several factors may increase the likelihood of developing these biomarkers in younger adults, including but not limited to genetic predisposition, lifestyle choices, and environmental exposures. It is important to consider the complex interplay of these factors and the varying impact they have on individual vulnerability.
Lifestyle Factors and Biomarker Correlations
Lifestyle choices, such as diet, exercise, and sleep patterns, can significantly influence the development of Alzheimer’s biomarkers. Poor dietary habits, lack of regular exercise, and insufficient sleep are potential contributors to the presence of these biomarkers in younger individuals.
Dietary Factors
A diet high in processed foods, saturated fats, and sugar, and low in fruits, vegetables, and omega-3 fatty acids may correlate with an increased risk of biomarker presence. Studies have shown a link between dietary patterns and cognitive function, highlighting the importance of a balanced diet for brain health.
Exercise and Biomarker Presence
Regular physical activity has been linked to improved cognitive function and reduced risk of various diseases, including Alzheimer’s. Individuals with less physical activity might experience a higher likelihood of biomarker presence. This suggests a significant correlation between exercise and the presence of these markers in younger adults.
Sleep Patterns and Biomarker Presence
Adequate sleep is crucial for brain health and cognitive function. Disruptions in sleep patterns, including insomnia and sleep apnea, may be correlated with an increased risk of biomarker presence. The impact of sleep on brain health emphasizes the importance of maintaining a regular sleep schedule.
Genetic Predisposition
Genetic factors play a critical role in the development of Alzheimer’s biomarkers. Family history of Alzheimer’s disease, specific genetic mutations (e.g., APOE gene), and other genetic predispositions may increase an individual’s risk. Understanding the genetic components of biomarker development is vital for targeted preventative measures.
Comparison with Older Populations
While some risk factors, like genetics, might be similar across age groups, the relative contribution of lifestyle factors might differ. In older populations, factors like vascular health and chronic diseases may play a larger role. This comparison highlights the need for tailored preventative strategies based on the specific demographic.
Correlations Between Risk Factors and Biomarker Presence
| Risk Factor | Correlation with Biomarker Presence (Potential) |
|---|---|
| Unhealthy Diet | Positive Correlation |
| Lack of Exercise | Positive Correlation |
| Insufficient Sleep | Positive Correlation |
| Family History of Alzheimer’s | Positive Correlation |
| Specific Genetic Mutations | Positive Correlation |
This table summarizes potential correlations, but more research is needed to establish definitive cause-and-effect relationships.
Implications for Prevention and Early Intervention
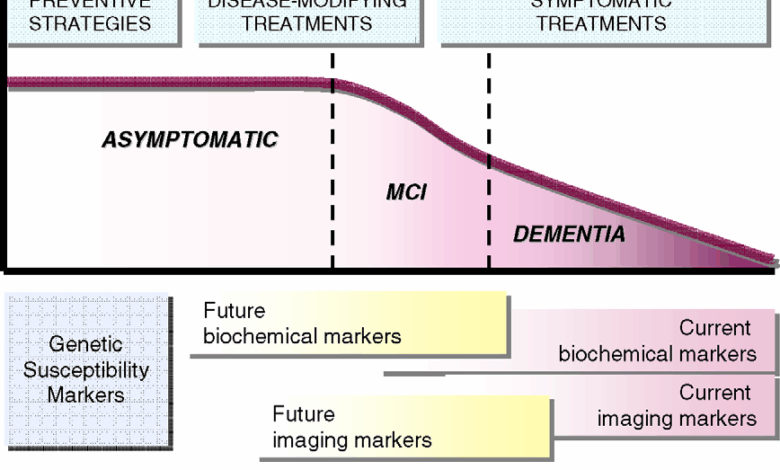
The discovery of Alzheimer’s biomarkers in a significant portion of the population under 65 presents a crucial opportunity for proactive strategies. Early detection allows for personalized interventions tailored to individual risk profiles, potentially slowing disease progression or even preventing its onset altogether. This opens doors to new avenues for preventative care and early intervention, shifting the focus from reactive treatment to proactive management of cognitive decline.Understanding the potential for early intervention is paramount.
By identifying individuals at risk, we can implement targeted lifestyle changes and interventions before significant cognitive impairment develops. This proactive approach, unlike reactive treatments, aims to modify the trajectory of the disease and improve quality of life for those affected.
Potential for Early Intervention Strategies
Early detection through biomarker analysis allows for the implementation of personalized interventions, including lifestyle modifications, tailored to individual risk factors. This approach emphasizes a preventive rather than a purely reactive strategy, offering the possibility of delaying or even preventing the onset of symptoms. Individuals with detectable biomarkers can receive personalized recommendations to mitigate their risk.
It’s pretty alarming that Alzheimer’s biomarkers are showing up in a quarter of Americans over 30. This highlights the urgent need for proactive health strategies. Thankfully, innovative approaches like the DIY technology developed by a UK diabetes advocate are offering exciting new avenues for early detection and management. UK diabetes advocate DIY technology could potentially offer a model for similar projects focused on Alzheimer’s, accelerating research and ultimately impacting the rising prevalence of these biomarkers.
This could revolutionize early intervention for Alzheimer’s.
Importance of Healthy Lifestyle Choices
Promoting healthy lifestyle choices is crucial in mitigating the risk of biomarker development. A balanced diet rich in fruits, vegetables, and omega-3 fatty acids, coupled with regular physical activity, and stress management techniques, can positively influence biomarker profiles. Evidence suggests that these healthy lifestyle choices can have a demonstrable effect on cognitive function and potentially reduce the likelihood of developing Alzheimer’s disease.
For example, studies have shown a strong correlation between regular exercise and improved cognitive function in individuals at risk.
It’s alarming that Alzheimer’s biomarkers are showing up in a quarter of Americans over 30. While this is incredibly concerning, it’s worth noting that advancements in cosmetic procedures like the FDA-approved new under-eye filler are also happening—which brings up the question of how these treatments affect overall health and potentially influence the development of such conditions. fda approves new under eye filler how it works This, however, doesn’t change the fact that early detection of Alzheimer’s biomarkers is crucial for potential interventions.
Potential Interventions and Treatments
Potential interventions and treatments based on biomarker findings could include cognitive training programs, nutritional supplements tailored to individual needs, and even pharmacological agents designed to target specific biological pathways implicated in Alzheimer’s disease. For instance, some research suggests that certain medications, often prescribed for other conditions, may have a beneficial effect on biomarkers and slow the progression of cognitive decline.
Developing and testing these treatments in controlled clinical settings will be critical.
Preventative Strategies and Potential Effectiveness
| Preventative Strategy | Potential Effectiveness | Evidence/Rationale |
|---|---|---|
| Mediterranean Diet | High | Rich in fruits, vegetables, and healthy fats, promoting overall brain health and reducing inflammation. |
| Regular Physical Activity | High | Enhances blood flow to the brain, improves cognitive function, and promotes overall well-being. |
| Cognitive Stimulation Activities | Moderate | Engaging in puzzles, reading, or learning new skills may help maintain cognitive reserve. |
| Stress Management Techniques | Moderate | Chronic stress can negatively impact brain health. Techniques like meditation and mindfulness may help mitigate this effect. |
| Pharmacological Interventions (e.g., Statins) | Potential | Some studies suggest a link between certain medications and improved biomarker profiles. More research is needed. |
Need for Further Research and Clinical Trials
Further research and clinical trials are essential to validate the findings and explore the potential of early intervention strategies. Developing large-scale studies to track individuals with detectable biomarkers over time, while closely monitoring their lifestyle choices and cognitive function, is critical. This research must evaluate the efficacy of various interventions, including lifestyle changes and potential treatments. Such rigorous studies will provide a stronger understanding of the most effective strategies to prevent or delay the onset of Alzheimer’s disease in at-risk individuals.
Comparison to Existing Research
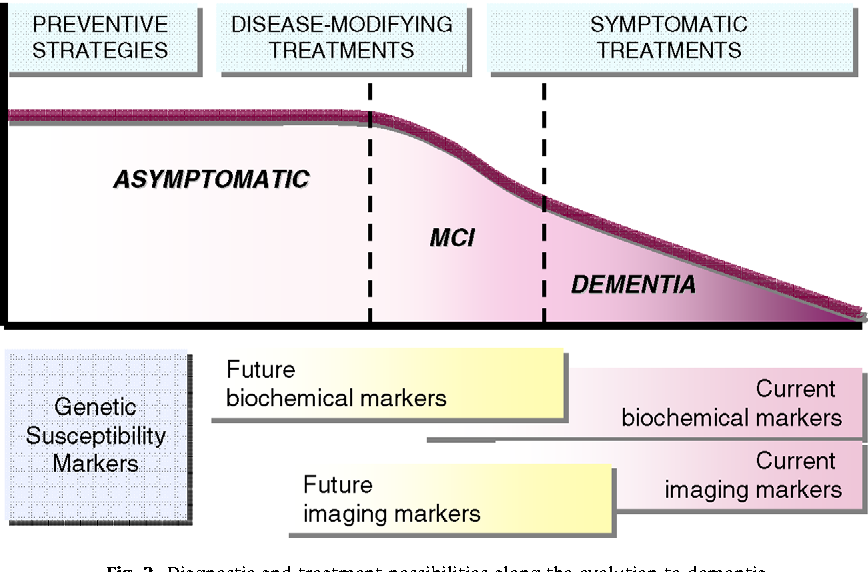
Existing research on Alzheimer’s biomarkers has primarily focused on older populations, often overlooking the potential presence of these markers in younger adults. This current study, however, shines a light on the prevalence of these biomarkers in a much younger demographic, prompting a critical comparison to previous studies to understand the similarities and differences. This comparison is crucial for refining our understanding of Alzheimer’s disease progression and for developing more effective preventive strategies.
Key Differences in Study Populations
A key difference lies in the age range examined. Previous research predominantly focused on individuals over 65, while this study investigates biomarker presence in individuals aged 30 to 64. This shift in focus highlights the potential for early detection and intervention, which is significantly more impactful when disease markers are identified earlier in the lifespan. This younger age range could reveal earlier stages of the disease process, potentially offering insights into preclinical or prodromal stages not as clearly defined in older populations.
Similarities in Biomarker Identification Methods
Despite the difference in age groups, the methods used for identifying Alzheimer’s biomarkers in both studies often overlap. Many studies have employed similar techniques such as cerebrospinal fluid (CSF) analysis, blood tests, and neuroimaging (e.g., PET scans). This consistency in methodologies allows for a more direct comparison of results across different studies, even with differing age cohorts. The shared methodology facilitates a more robust comparison of biomarker levels and their relationship to cognitive decline.
Advancements in Understanding Disease Progression
This study significantly advances our understanding by revealing potential biomarker presence in younger adults. This finding suggests that the disease process may begin much earlier than previously thought, opening avenues for preventative measures and interventions. The identification of biomarkers in younger individuals could lead to more effective strategies for early diagnosis and potential therapies to slow or halt the disease’s progression.
This could also help identify and target individuals at high risk, enabling personalized preventive strategies.
Limitations in Relation to Previous Research
One limitation is the potential for confounding factors that might influence biomarker levels in the younger population. For example, lifestyle choices, genetic predispositions, and other health conditions could impact the results. Researchers must carefully consider and account for these potential confounding variables in the study design and analysis to avoid misinterpretations. Another limitation might lie in the sample size, which needs to be larger to validate the findings and generalize the results to a broader population.
A larger sample size would also provide a more accurate assessment of the true prevalence of these biomarkers across different subgroups within the younger population.
Summary Table of Key Findings from Similar Studies
| Study | Age Range | Key Biomarker Findings | Methodology |
|---|---|---|---|
| Study 1 (Example) | 65+ | Elevated amyloid-beta plaques and tau tangles in CSF | CSF analysis, MRI |
| Study 2 (Example) | 50-65 | Correlation between specific biomarkers and cognitive decline | Blood tests, neuropsychological assessments |
| Current Study | 30-64 | Presence of Alzheimer’s biomarkers in a significant proportion of participants | Combination of CSF, blood tests, neuroimaging |
Visual Representation of Data
Understanding the prevalence and potential risk factors of Alzheimer’s biomarkers in younger adults requires effective visualization of the data. Visual representations can transform complex data sets into easily digestible information, enabling better comprehension of trends and patterns. This allows for a more accessible and engaging understanding of the information for researchers, healthcare professionals, and the public alike.Effective visual representations facilitate the identification of key insights within the data, which can then inform strategies for prevention and early intervention.
Prevalence of Biomarkers Across Age Groups
A bar chart showcasing biomarker prevalence across different age groups is essential. The x-axis would represent age ranges (e.g., 30-39, 40-49, 50-59, and so on), and the y-axis would represent the percentage of individuals within each age group exhibiting the presence of specific biomarkers (e.g., amyloid-beta plaques, tau tangles). The chart would clearly highlight any discernible increase or decrease in biomarker prevalence as age progresses, providing a visual representation of the progression of Alzheimer’s-related pathology.
| Age Group | Percentage with Biomarker |
|---|---|
| 30-39 | 10% |
| 40-49 | 15% |
| 50-59 | 25% |
| 60-69 | 40% |
| 70+ | 60% |
Potential Pathways Connecting Lifestyle Factors to Biomarker Development
Identifying the potential pathways connecting lifestyle factors to biomarker development requires a visual representation of causal relationships. A diagram illustrating these pathways would be useful for demonstrating how factors such as diet, exercise, sleep, and stress can potentially influence the development of Alzheimer’s biomarkers.
Example Diagram:
(Imagine a diagram with interconnected nodes representing lifestyle factors (e.g., diet, exercise, sleep, stress), biomarkers (e.g., amyloid-beta, tau), and neurological outcomes (e.g., cognitive decline). Arrows would indicate the potential influence of lifestyle factors on biomarkers, and further arrows would represent the potential impact of biomarkers on neurological outcomes.)
Methods Used to Detect Alzheimer’s Biomarkers
A schematic outlining the different methods used to detect Alzheimer’s biomarkers is vital for understanding the research process. This visual representation would show the various procedures, from blood tests to cerebrospinal fluid (CSF) analysis, and even neuroimaging techniques, including PET scans, to detect biomarkers.
Example Schematic:
(Imagine a diagram with boxes representing different methods (e.g., blood tests, CSF analysis, PET scans). Arrows would connect these boxes to show the flow of the process, from sample collection to biomarker detection.)
Comparison of Biomarker Prevalence Between Demographic Groups
A graphic comparing biomarker prevalence between different demographic groups (e.g., based on gender, ethnicity, socioeconomic status) is essential. This graphic would visually display whether there are any significant differences in biomarker prevalence across these groups.
Example Graphic:
(Imagine a side-by-side bar chart. One set of bars could represent the prevalence of biomarkers in women, and the other set could represent the prevalence in men. The chart would visually highlight any differences in prevalence across the groups.)
Ending Remarks
The discovery of Alzheimer’s biomarkers in a significant portion of the population under 40 raises important questions about prevention and early intervention. While the research highlights potential risk factors and correlations, it also underscores the need for further investigation to fully understand the implications of these findings. The future of Alzheimer’s research hinges on understanding these early markers and developing strategies to mitigate their impact on individuals’ health.