Antibodies producing faulty results is a serious concern in medical diagnostics. These errors can lead to misdiagnosis, inappropriate treatments, and anxiety for patients. Understanding the origins of these faulty results, the types of errors, and the consequences they bring is crucial. Pre-analytical, analytical, and post-analytical factors all play a role in the accuracy of antibody testing. This blog post will explore these factors in detail, highlighting potential issues and offering strategies to mitigate these problems.
From sample handling to laboratory equipment, we’ll cover a wide range of aspects, including the different types of inaccurate results like false positives, false negatives, and inconsistencies. We’ll also look at the impact of these errors on patient care, outlining the potential for inappropriate treatment, delayed diagnosis, and unnecessary interventions. Finally, we’ll explore the importance of quality control measures, proper interpretation, and effective communication in reporting antibody test results to prevent these errors and ensure accurate patient care.
Faulty Antibody Result Origins
Antibody testing, while crucial for diagnosis and monitoring, can sometimes produce inaccurate results. Understanding the potential sources of error is vital for clinicians to interpret results correctly and avoid misdiagnosis or inappropriate treatment. This blog post delves into the pre-analytical, analytical, and post-analytical factors that can contribute to faulty antibody readings, equipping you with the knowledge to critically evaluate these results.
Pre-analytical Factors
Pre-analytical errors occur before the actual analysis of the sample. These errors are often the most significant contributors to inaccurate results, as they can compromise the integrity of the sample itself. Proper sample collection, handling, and storage are critical for obtaining reliable antibody test results. Inadequate sample collection techniques, incorrect labeling, or improper storage conditions can lead to significant variations in the measured antibody levels.
- Sample Collection Errors: Incorrect collection techniques can introduce contaminants or alter the concentration of the antibody in the sample. For instance, insufficient blood volume or improper mixing of the collected blood can lead to inaccurate readings. Hemolysis (breakdown of red blood cells), lipemia (excess fat), or icterus (jaundice) can also interfere with the test. Careful attention to standardized collection protocols is paramount.
- Sample Handling and Storage: Timely processing and appropriate storage are crucial. Delayed processing or inappropriate storage temperatures can alter the stability of the antibodies. This may lead to either falsely elevated or falsely decreased readings. Proper storage procedures should be meticulously followed, and strict time limits for sample processing must be adhered to.
Analytical Factors
Analytical errors occur during the actual testing process. These errors can be attributed to equipment malfunctions, procedural deviations, or limitations of the assay itself.
- Equipment Malfunctions: Calibration issues, faulty instrumentation, or inadequate maintenance of laboratory equipment can all contribute to inaccurate readings. For example, a spectrophotometer not properly calibrated can produce inconsistent absorbance readings, affecting the antibody concentration calculation. Regular maintenance and quality control checks are essential to mitigate these errors.
- Procedural Deviations: Inconsistent or incorrect laboratory procedures can lead to unreliable results. This includes errors in reagent preparation, incorrect pipetting volumes, or improper mixing of reagents. Adherence to standardized operating procedures (SOPs) and thorough training for laboratory personnel are critical.
Post-analytical Factors
Post-analytical errors encompass the steps taken after the analysis is complete. These errors often relate to the interpretation and reporting of results.
- Data Entry Errors: Mistakes during data entry or transcription of results can lead to misinterpretations. Double-checking data and utilizing automated systems for data entry can minimize these errors.
- Reporting Errors: Incorrect or incomplete reporting of results can lead to misdiagnosis. For example, a failure to include critical clinical information, such as patient history or symptoms, can significantly impact the interpretation of antibody results. Results should be interpreted within the context of the complete clinical picture.
Potential Errors in Antibody Testing
| Stage | Potential Error | Impact |
|---|---|---|
| Pre-analytical | Inadequate sample collection, incorrect labeling, improper storage, hemolysis, lipemia, icterus | Inaccurate antibody concentration measurement, potentially leading to misdiagnosis. |
| Analytical | Equipment malfunction (e.g., spectrophotometer calibration issues), procedural deviations (e.g., incorrect reagent preparation), assay limitations | Inconsistent or inaccurate antibody measurements. |
| Post-analytical | Data entry errors, reporting errors (missing clinical information), lack of result validation | Misinterpretation of results, delayed diagnosis, or inappropriate treatment. |
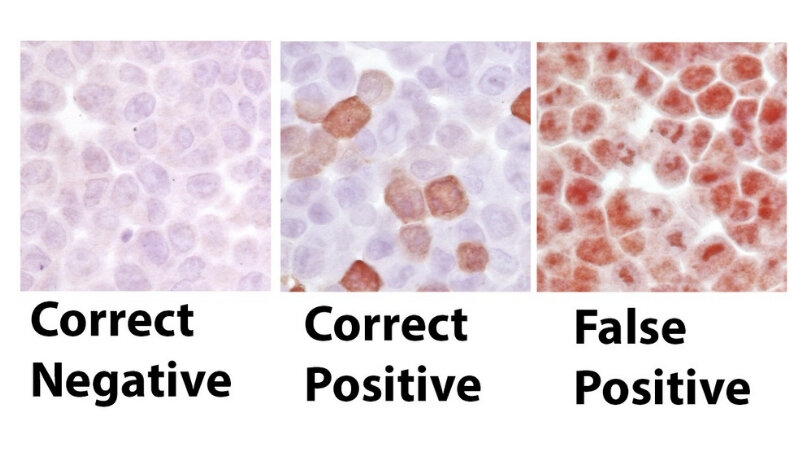
Types of Faulty Antibody Results
Antibody tests, while powerful diagnostic tools, are not infallible. Errors in these tests can lead to misdiagnosis and inappropriate treatment. Understanding the different types of faulty results is crucial for clinicians to interpret the data accurately and make informed decisions. This exploration will delve into false positives, false negatives, and inconsistent results, examining their implications in patient care.
False Positive Results
False positive antibody results occur when the test indicates the presence of antibodies when, in reality, they are not present. This can be due to several factors, including cross-reactivity of the test reagents with other substances, or even pre-existing conditions that mimic the target antibody.A significant concern with false positives is the unnecessary anxiety and potential for unwarranted treatment that they can generate.
For instance, a false positive for HIV antibodies could lead to significant psychological distress and potentially invasive follow-up procedures. Furthermore, the over-diagnosis of conditions can result in unnecessary medication use and associated side effects.
False Negative Results
False negative antibody results occur when the test indicates the absence of antibodies when, in fact, they are present. This can stem from several factors, such as low antibody levels, insufficient sample collection, or the test’s inability to detect specific antibody types. The implications of a false negative are equally serious. A false negative for rubella antibodies, for example, could lead to the delayed diagnosis and treatment of the infection, potentially resulting in severe complications.
Inconsistent Results
Inconsistent antibody results present a particular challenge. These results fluctuate over time, or from different samples taken from the same patient. They often require careful review and repeated testing to determine if the initial results were a genuine measurement or an anomaly.Inconsistent results can be attributed to various factors, including the patient’s health status, changes in antibody levels, or laboratory errors.
For instance, an inconsistent result for Lyme disease antibodies could make it difficult to establish a clear diagnosis and guide treatment decisions.
Summary Table of Faulty Antibody Results
| Type | Description | Impact |
|---|---|---|
| False Positive | The test indicates the presence of antibodies when they are not present. | Unnecessary anxiety, unwarranted treatment, potential for over-diagnosis. |
| False Negative | The test indicates the absence of antibodies when they are present. | Delayed diagnosis, inadequate treatment, potential for severe complications. |
| Inconsistent | Results fluctuate over time or from different samples, potentially indicative of an anomaly or actual change in antibody levels. | Difficulty in establishing a clear diagnosis, challenges in guiding treatment. |
Consequences of Faulty Antibody Results
Misinterpreting antibody test results can have serious repercussions for patient care. These tests, while valuable diagnostic tools, are not without their limitations. Errors in testing methodology, sample handling, or interpretation can lead to inaccurate diagnoses, inappropriate treatments, and significant distress for patients. Understanding the potential consequences is crucial for healthcare professionals to ensure patient safety and well-being.Faulty antibody test results can have a cascading effect, impacting the entire trajectory of a patient’s care.
The consequences extend beyond simply an incorrect diagnosis, potentially leading to delays in treatment, unnecessary interventions, and even harm. This underscores the critical importance of rigorous quality control measures and meticulous interpretation of antibody test results.
Adverse Effects on Patient Care
Inaccurate antibody test results can lead to a variety of adverse effects on patient care. These effects range from the emotional distress of misdiagnosis to the potentially more serious consequences of inappropriate treatment or delayed interventions. The consequences are compounded when the results are misinterpreted, leading to further complications.
Inappropriate Treatment or Delayed Diagnosis
A faulty antibody result can lead to inappropriate treatment, either by failing to initiate necessary treatment or by administering treatments that are ineffective or even harmful. Similarly, a delayed diagnosis can exacerbate the disease process, potentially leading to irreversible damage or complications. For example, a false-negative result for a critical illness could delay crucial interventions, while a false-positive result could lead to unnecessary anxiety and potentially harmful treatments.
The delay in initiating appropriate treatment for infectious diseases can have significant implications for disease progression, transmission, and ultimately, patient outcomes.
Unnecessary Interventions and Patient Anxiety
Misinterpreted antibody test results can lead to unnecessary interventions and cause significant anxiety for patients. A false-positive result for a condition might trigger a cascade of tests and procedures, leading to financial burdens and emotional distress. This is particularly concerning for conditions with significant psychological implications. The emotional toll of a potential illness, even when unfounded, is substantial.
Anxiety about a disease can manifest in a multitude of ways, impacting quality of life.
Examples of Negative Patient Outcomes
Several cases demonstrate the detrimental effects of inaccurate antibody test results. For instance, a patient with a suspected infectious disease might be delayed in receiving appropriate treatment if the initial antibody test is incorrectly interpreted as negative. This delay could allow the disease to progress, potentially causing severe complications or even death. Similarly, a false-positive result for an autoimmune condition could lead to unnecessary and potentially harmful immunosuppressive therapies.
These examples highlight the necessity for rigorous quality control measures and meticulous interpretation of antibody test results.
Legal and Ethical Implications
Faulty antibody results can have profound legal and ethical implications. Healthcare providers may face legal ramifications for misdiagnosis or delayed treatment. Additionally, patients might pursue legal action for damages caused by these errors. The ethical implications are equally significant, requiring healthcare providers to maintain the highest standards of accuracy and responsibility in their diagnostic practices. Transparency in reporting errors and implementing corrective measures is critical to upholding ethical standards and building patient trust.
Antibody tests sometimes give inaccurate readings, leading to confusion and potentially incorrect diagnoses. Recent research suggests that certain factors, like the specific type of antibody or the timing of the test, might play a role in these faulty results. Interestingly, a new study on Celebrex suggests it might be safer for the heart than previously thought, celebrex safer for heart than thought.
This could influence how doctors interpret antibody results in patients taking this medication, highlighting the importance of considering the broader clinical picture when evaluating antibody-based diagnostics.
The following list details potential legal and ethical issues that can arise from faulty antibody test results:
- Medical Malpractice Claims: If a patient suffers harm due to a misdiagnosis or delayed treatment stemming from a faulty antibody test, they may file a medical malpractice claim against the healthcare provider(s) involved. The severity of the harm and the evidence of negligence will determine the outcome of such claims.
- Breach of Duty: Healthcare professionals have a duty of care to their patients. Failing to accurately interpret antibody test results could constitute a breach of this duty, leading to legal consequences.
- Ethical Considerations: The ethical implications of faulty antibody results encompass issues of patient autonomy, informed consent, and the responsibility to provide accurate and timely diagnoses. Healthcare providers must ensure they are adhering to established ethical standards.
Mitigation Strategies for Faulty Antibody Results: Antibodies Producing Faulty Results
Antibody testing, while crucial for disease diagnosis and surveillance, is susceptible to errors. These errors can lead to misdiagnosis, inappropriate treatment, and public health concerns. Implementing robust quality control measures is paramount to minimizing these issues and ensuring reliable results. A well-structured quality assurance program plays a critical role in maintaining the accuracy and validity of antibody test outcomes.
Quality Control Measures in Antibody Testing
Quality control (QC) measures are essential components of a reliable antibody testing procedure. These measures ensure the accuracy and precision of the test results. QC involves establishing and maintaining standards for reagents, equipment, and personnel procedures. Consistent adherence to these standards helps minimize errors and maintain the validity of test results.
- Reagent Validation: Thorough validation of antibody reagents is crucial. This includes testing for purity, specificity, and sensitivity, ensuring they react accurately with the target analyte. Validation protocols should be documented and regularly reviewed to maintain reagent integrity.
- Equipment Calibration: Calibration of laboratory equipment, such as spectrophotometers and automated analyzers, is critical for precise measurements. Regular calibration checks, using certified standards, are essential to maintain instrument accuracy. Deviation from established calibration parameters should trigger immediate investigation and corrective actions.
- Personnel Training: Proper training of laboratory personnel is fundamental to minimizing human error. Comprehensive training programs should cover all aspects of antibody testing, including sample handling, reagent preparation, instrument operation, and result interpretation. Training materials should be readily accessible, and ongoing competency assessments should be performed to ensure consistent proficiency.
Validation Procedures for Laboratory Equipment and Reagents
Validation procedures ensure that laboratory equipment and reagents meet the necessary specifications for accurate and reliable antibody testing. This process involves a series of steps designed to demonstrate that the equipment and reagents perform as expected under various conditions. The validation protocols should be documented and reviewed periodically.
Antibodies sometimes produce inaccurate readings, leading to frustrating misdiagnoses or treatment adjustments. Thankfully, innovative solutions like the DIY technology developed by a UK diabetes advocate are helping to improve accuracy in monitoring blood glucose levels. This UK diabetes advocate’s resourceful approach to diabetes management, detailed in this fascinating piece UK diabetes advocate DIY technology , highlights the potential for personalized and potentially more reliable antibody-based testing.
Ultimately, these kinds of developments are crucial in addressing the issues surrounding antibody-based results.
- Reagent Characterization: Characterizing reagents is a critical aspect of validation. This includes determining the specificity, sensitivity, and linearity of the reagents, and defining the range of concentrations for which they perform optimally. This process establishes the reliable range for the antibody testing and helps avoid misleading results from outside the defined range.
- Equipment Performance Testing: Equipment performance testing should cover a wide range of conditions. This includes testing at different temperatures, humidity levels, and sample volumes. It should also assess the precision and accuracy of the equipment across various sample types. Documentation of these tests and maintenance records are vital for quality control.
Importance of Proper Training for Laboratory Personnel
Adequate training for laboratory personnel is essential to prevent errors in antibody testing. A well-trained workforce is better equipped to handle various situations, maintain consistent protocols, and identify potential issues promptly. This minimizes errors during sample handling, reagent preparation, instrument operation, and result interpretation.
Sometimes antibodies just aren’t cutting it, producing faulty results that can throw off diagnoses. It’s a complex issue, but consider this: flushing a toilet can release viral droplets, impacting air quality and potentially influencing test results. Learning about how simple actions like this affect our environment and potentially impact antibody testing accuracy is crucial. So, while antibodies might not be performing perfectly, understanding the surrounding factors is equally important for getting reliable results.
Check out this fascinating article on flushing a toilet can release viral droplets what to know for more insights.
- Comprehensive Training Programs: Comprehensive training programs should cover the entire antibody testing process, from sample collection to result reporting. This includes theoretical knowledge, practical exercises, and ongoing monitoring of performance.
- Hands-on Experience: Hands-on experience is crucial for laboratory personnel to gain practical skills in handling various equipment and reagents. Simulated scenarios and practical exercises can reinforce theoretical knowledge and ensure proficiency in antibody testing techniques.
Flowchart of Comprehensive Quality Assurance Program
A comprehensive quality assurance program for antibody testing involves a systematic approach to ensure accuracy and reliability. The flowchart below Artikels the key steps involved in such a program.
- Initial Reagent Validation: This involves verifying the quality and characteristics of the reagents used in the antibody testing process.
- Equipment Calibration: Regular calibration of the laboratory equipment is essential to maintain accurate measurements.
- Personnel Training: Training programs should be implemented and regularly reviewed to maintain staff proficiency.
- Daily Quality Control Checks: Implementing daily QC checks to ensure instrument functionality and reagent performance.
- Regular Performance Testing: Regular performance testing of laboratory equipment and reagents is crucial to identify and address potential issues early on.
- Review and Feedback: Regular review of the results and feedback from staff members to identify areas for improvement.
- Corrective Actions: Taking corrective actions based on the feedback and reviews.
Regular Performance Testing of Laboratory Equipment and Reagents
Regular performance testing is vital for maintaining the accuracy and reliability of antibody testing. This process involves regularly checking the equipment and reagents for proper function. This ensures that the testing procedures are up to date and meet the required standards.
- Frequency of Testing: The frequency of performance testing should be determined based on the specific equipment and reagents, and the nature of the tests being performed. Equipment manufacturers’ recommendations and established lab protocols should be considered.
- Monitoring and Analysis: Monitoring and analyzing the results of performance testing are essential for identifying trends and patterns. This helps to proactively address potential issues before they impact the accuracy of test results.
Interpretation and Reporting of Antibody Results
Antibody tests, while valuable diagnostic tools, require meticulous interpretation to avoid misdiagnosis. Proper interpretation hinges on a thorough understanding of the test’s limitations, the patient’s clinical presentation, and the overall context of the patient’s health history. A nuanced approach that integrates laboratory findings with clinical information is paramount for accurate results.Accurate interpretation and reporting of antibody test results are critical to ensuring appropriate patient management and treatment decisions.
This process involves a combination of laboratory expertise and clinical judgment, requiring careful consideration of various factors. A thorough understanding of best practices is essential for reliable and accurate results.
Best Practices for Interpreting Antibody Test Results
Interpreting antibody test results requires a systematic approach. It’s crucial to consider the specific test used, the reference range for the particular antibody, and the patient’s clinical history. A thorough understanding of the test methodology and its limitations is essential.
Considering Clinical Context Alongside Laboratory Findings
Clinical context is paramount in interpreting antibody test results. A patient’s symptoms, medical history, and exposure history significantly influence the interpretation of laboratory findings. For example, a positive result for antibodies against a specific virus might be expected in a patient with a confirmed infection. However, the same result in a patient with no relevant exposure history or symptoms warrants further investigation.
Considering the clinical context ensures a more accurate and nuanced interpretation of the antibody test results.
Evaluating Antibody Titers and Patterns for Accurate Interpretation
Antibody titers represent the concentration of antibodies in a patient’s serum. Higher titers often suggest a more recent or active infection. The pattern of antibody responses, such as the presence of IgM and IgG antibodies at different time points, can provide crucial insights into the infection’s progression. Understanding the expected kinetics of antibody development for a particular infection is essential.
For example, an acute infection might show a surge in IgM antibodies followed by a rise in IgG antibodies. Careful evaluation of these patterns can aid in distinguishing between past, current, and potential future infections.
Examples of Clinical Scenarios Where Multiple Tests are Used to Evaluate Antibody Responses, Antibodies producing faulty results
In complex cases, multiple antibody tests are often employed to gain a comprehensive understanding of a patient’s immune response. For instance, in assessing a patient with suspected chronic viral infection, the physician might use a combination of antibody tests to determine if there has been prior exposure and if the infection is currently active. Different tests may target different stages of an infection or different parts of the immune response.
This approach provides a more complete picture of the immune system’s response, aiding in diagnosis and prognosis.
Need for Proper Documentation of Antibody Test Results and Their Interpretation
Thorough documentation of antibody test results is essential for maintaining accurate patient records. This documentation should include the date and time of the test, the specific antibody tested for, the result obtained, the reference range used, and the clinical context. The interpretation of the results, including any conclusions drawn from the findings, should also be meticulously documented. This detailed documentation facilitates effective communication among healthcare providers and allows for tracking of antibody responses over time.
Clearly Communicating Test Results to Healthcare Providers and Patients
Clear and concise communication of antibody test results is crucial for effective patient management. Results should be presented in a way that is easily understood by both healthcare providers and patients. The language used should be non-technical and avoid jargon. Providing context and explanation of the findings is essential. For instance, explaining the significance of a positive result in the context of the patient’s symptoms and medical history can improve understanding and facilitate informed decision-making.
Last Recap
In conclusion, antibody testing plays a critical role in diagnosing and managing various health conditions. However, the potential for faulty results necessitates a thorough understanding of the entire process, from sample collection to result interpretation. By recognizing the potential sources of error, implementing robust quality control measures, and prioritizing accurate interpretation, we can minimize the adverse consequences of faulty antibody results.
This detailed analysis underscores the importance of meticulous attention to detail and adherence to best practices in antibody testing to ensure the reliability and safety of patient care.
