Brain cancer vaccine clinical trial sets the stage for a potentially revolutionary approach to treating this devastating disease. Current treatments often have limited effectiveness, leaving many patients facing challenging prognoses. This trial explores the use of vaccines to stimulate the body’s immune response against brain cancer cells, offering a novel strategy for prevention and treatment. Different types of vaccines are being tested, each with its own mechanism of action and target antigens.
We’ll delve into the specifics of these trials, including their design, potential benefits, risks, and the ongoing progress of research.
The clinical trials for brain cancer vaccines encompass a range of phases, from initial safety assessments to large-scale studies. Each phase plays a critical role in evaluating the vaccine’s efficacy and safety profile before potential widespread use. This article explores the methodology behind these trials, including the selection criteria for participants, and the potential hurdles that researchers must overcome.
Understanding the details of these trials is crucial for anyone interested in this groundbreaking field.
Introduction to Brain Cancer Vaccines
Brain cancer, a devastating disease affecting the central nervous system, presents a significant challenge for modern medicine. Currently, treatment options, while sometimes effective, often come with significant side effects. The existing treatments primarily focus on surgery, radiation therapy, and chemotherapy, each with limitations in terms of efficacy and potential for long-term harm. This necessitates the exploration of novel approaches, and brain cancer vaccines represent a promising avenue for future therapies.The rationale behind developing brain cancer vaccines stems from the understanding that the immune system, under the right circumstances, can recognize and eliminate cancer cells.
Exciting news on the brain cancer vaccine clinical trial front! Researchers are pushing boundaries, and it’s inspiring to see how innovative treatments are emerging. The pandemic, however, also surprisingly fostered a surge in virtual rehabilitation options, like how the pandemic led to the rise of virtual rehab , which could potentially revolutionize patient care for many conditions, including brain cancer.
This highlights the interconnectedness of various fields, and I’m hopeful that these developments will eventually lead to better outcomes in the brain cancer vaccine clinical trial.
Traditional cancer treatments often target rapidly dividing cells, impacting both cancerous and healthy cells, leading to side effects. Vaccines, by stimulating the immune system to specifically target cancer cells, offer the potential for a more precise and less harmful approach to treatment. This targeted approach could offer a more individualized and potentially less damaging therapeutic strategy for cancer patients.
Different Types of Brain Cancer Vaccines in Clinical Trials
Various vaccine strategies are being explored in clinical trials, each with its own approach to stimulating the immune response against brain cancer. These vaccines aim to equip the immune system to identify and destroy cancer cells by presenting specific tumor antigens to immune cells.
Comparison of Vaccine Types
| Vaccine Type | Mechanism of Action | Target Antigen | Phase of Trial |
|---|---|---|---|
| Peptide-based vaccines | These vaccines utilize short chains of amino acids (peptides) derived from tumor antigens. These peptides are presented to the immune system, stimulating an immune response against the specific tumor cells. | Specific proteins expressed on the surface of brain tumor cells. | Mostly in Phase I/II |
| Whole-cell vaccines | These vaccines use a whole tumor cell, or a portion of it, as an antigen. This allows the immune system to recognize a broader range of tumor-associated antigens. | A range of tumor proteins and molecules on the cell surface. | Phase I/II trials are ongoing |
| DNA vaccines | These vaccines introduce DNA encoding tumor antigens into the body. The body’s cells then produce the antigens, stimulating an immune response. | Specific proteins unique to brain tumors. | Some in Phase I/II |
| Viral vector vaccines | These vaccines use viruses as vectors to deliver tumor antigens to the immune system. The virus is modified to carry the antigen, allowing for targeted presentation to immune cells. | Tumor-specific antigens. | Phase I/II trials ongoing |
The table above provides a snapshot of the different approaches being explored in clinical trials. Further research and development are crucial to optimize the efficacy and safety of these vaccine strategies. The choice of vaccine type and its specific mechanism of action often depend on the particular type of brain tumor and the characteristics of the patient.
Clinical Trial Design and Methodology
Unveiling the intricate process of testing a brain cancer vaccine requires meticulous planning and execution. A clinical trial acts as a crucial step in evaluating the safety and efficacy of a new treatment, meticulously assessing its impact on patients. This process, characterized by careful design and adherence to ethical guidelines, ensures that any new intervention is thoroughly evaluated before it reaches the wider population.
The clinical trial methodology, particularly the phases, inclusion/exclusion criteria, and overall design, is paramount to ensuring the safety and efficacy of the treatment.
Phases of Clinical Trials
Clinical trials are typically conducted in phases, each with distinct objectives and expected outcomes. These phases are designed to progressively evaluate the safety and effectiveness of the treatment. Understanding these phases is crucial to grasping the rigorous process involved.
- Phase 1: This initial phase focuses primarily on safety. A small group of patients receives the vaccine to assess its tolerability and identify potential side effects. Researchers carefully monitor participants for any adverse reactions. This phase also helps determine the appropriate dosage and administration route.
- Phase 2: Building on the safety data from Phase 1, Phase 2 expands the trial to a larger group of patients. The primary goal is to further evaluate the safety and efficacy of the vaccine. Researchers begin to look at whether the vaccine is having the intended effect on the cancer cells. Different doses and administration schedules might be tested in this phase.
- Phase 3: In this pivotal phase, the vaccine is compared to existing treatments or a placebo in a larger population. This phase is designed to confirm the vaccine’s efficacy and assess its overall benefit in a wider context. The trial data collected in this phase is crucial for regulatory approval.
Inclusion and Exclusion Criteria
Careful selection of participants is crucial to ensure the validity of the clinical trial results. Inclusion and exclusion criteria define the characteristics of the patients who can participate in the trial.
- Inclusion Criteria: These criteria specify the characteristics that potential participants must possess to be eligible for the trial. Examples include specific types of brain tumors, stage of the disease, and overall health status. Matching patients with similar characteristics ensures meaningful comparisons during the trial.
- Exclusion Criteria: These criteria identify the characteristics that would disqualify a potential participant. Examples include pre-existing medical conditions that might interact with the vaccine, or other treatments that could confound the results. By excluding individuals with confounding factors, researchers can isolate the effects of the vaccine.
Trial Design Table
The following table Artikels the different phases of the clinical trial, their primary objectives, expected outcomes, and approximate duration.
Exciting news on the brain cancer vaccine clinical trial front! Researchers are making strides in developing potential treatments, but there’s still a long way to go. Meanwhile, I’ve been thinking about how beneficial activities like swimming for children with asthma can be for overall health. The physical benefits are undeniable, and hopefully, this type of focus on healthy habits will contribute to the positive progress being made in the brain cancer vaccine clinical trial.
| Trial Phase | Objectives | Expected Outcomes | Duration (Estimated) |
|---|---|---|---|
| Phase 1 | Assess safety, identify appropriate dosage, and determine administration route. | Identification of potential side effects, optimal dosage range, and administration method. | 6-12 months |
| Phase 2 | Evaluate safety and preliminary efficacy, explore different treatment strategies. | Further characterization of side effects, assessment of vaccine’s impact on tumor growth, and refinement of treatment strategies. | 12-18 months |
| Phase 3 | Compare the vaccine to existing treatments or a placebo, confirm efficacy and overall benefit. | Confirmation of the vaccine’s effectiveness, comparison with existing therapies, and identification of the optimal treatment strategy. | 2-4 years |
Potential Benefits and Risks
A brain cancer vaccine, a revolutionary approach to fighting this devastating disease, holds immense promise for improving patient outcomes. While the potential benefits are significant, it’s crucial to acknowledge the potential risks and carefully weigh them against the hope of a more effective treatment. This section delves into the anticipated advantages, the possible side effects, and the distinctions between various vaccine strategies.Understanding the intricacies of both the potential benefits and the potential risks is vital for making informed decisions about participation in clinical trials and for fostering public understanding of this groundbreaking research.
Potential Benefits of Brain Cancer Vaccines
Brain cancer vaccines aim to stimulate the body’s immune system to recognize and attack cancer cells. This targeted approach, if successful, could lead to improved survival rates and a reduced risk of recurrence. Success in clinical trials of vaccines for other cancers offers hope for similar outcomes in the fight against brain tumors. The precise mechanisms by which these vaccines achieve these outcomes remain under investigation, but preliminary data suggests promising results.
Increased immune response to tumor cells may lead to a stronger anti-tumor effect.
Potential Risks and Side Effects, Brain cancer vaccine clinical trial
Any medical intervention carries potential risks, and brain cancer vaccines are no exception. Possible side effects could range from mild discomfort to more severe complications. The nature and severity of these side effects are expected to vary based on the specific vaccine and the individual’s response to the treatment.
Comparison of Different Vaccine Approaches
Various approaches to brain cancer vaccines are currently under development. Each method has its own set of potential advantages and disadvantages. For example, vaccines based on tumor-associated antigens might elicit a more targeted immune response but might also pose a higher risk of autoimmune reactions. Vaccines incorporating immune checkpoint inhibitors could enhance the immune response but might require careful monitoring to prevent adverse reactions.
This section focuses on the inherent variability in approaches.
Table of Potential Side Effects
| Side Effect | Severity Level | Frequency | Mechanism |
|---|---|---|---|
| Injection site reactions (pain, redness, swelling) | Mild | Common | Local inflammatory response to the injection |
| Fever | Mild to Moderate | Possible | Immune system activation |
| Fatigue | Mild to Moderate | Possible | General systemic response to the treatment |
| Headache | Mild to Moderate | Possible | Potential inflammatory response in the brain or related to immune system activation. |
| Nausea and Vomiting | Mild to Moderate | Possible | Systemic effects of immune stimulation or inflammatory response |
| Autoimmune reactions (e.g., inflammation of the nervous system) | Severe | Uncommon | Immune system mistakenly attacking healthy tissues |
| Allergic reactions | Severe | Rare | Hypersensitivity response to vaccine components |
Careful monitoring and management of potential side effects are crucial aspects of brain cancer vaccine trials.
Current Status and Future Directions: Brain Cancer Vaccine Clinical Trial
The quest for a brain cancer vaccine is a marathon, not a sprint. While significant progress has been made in basic research and pre-clinical trials, translating this into effective clinical treatments remains a considerable hurdle. The complexity of the brain’s immune system and the intricate nature of tumor development contribute to the challenge. However, ongoing research offers glimpses into potential breakthroughs and innovative strategies for the future.
Current Progress in Clinical Trials
Clinical trials evaluating brain cancer vaccines are still relatively nascent. A significant portion of research is focused on Phase I and II trials, primarily assessing safety and identifying optimal dosages and delivery methods. These trials often involve smaller patient populations and aim to pinpoint the most promising vaccine formulations and strategies for subsequent phases. Early results are often promising in terms of safety profiles and potential immune responses, but large-scale efficacy data is still limited.
Challenges in Developing Effective Vaccines
Developing effective brain cancer vaccines faces numerous obstacles. The blood-brain barrier (BBB), a protective barrier in the brain, limits the access of immune cells and vaccine components to tumor cells. Moreover, brain tumors often exhibit heterogeneity, meaning they can vary significantly in their genetic makeup and cellular composition. This variability poses a challenge for designing a vaccine that can target all types of brain tumors effectively.
Furthermore, the immune response in the brain is different from other parts of the body, requiring specialized strategies to elicit an effective anti-tumor response. Finally, the development of effective and sustained immune responses against brain tumors is a complex undertaking.
Animal Studies and Pre-clinical Results
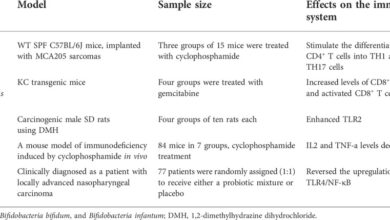
Animal models play a crucial role in testing the efficacy and safety of brain cancer vaccines before human trials. Pre-clinical studies using various animal models, such as mice and rats, have demonstrated promising results in terms of inducing anti-tumor immune responses and tumor regression. For instance, studies utilizing genetically modified mice with human brain tumor cells have shown that certain vaccine formulations can stimulate T-cell responses, leading to tumor shrinkage.
These findings are encouraging and suggest that the chosen vaccine strategies may hold promise for clinical applications. Detailed analyses of these animal studies provide vital insights into the mechanism of action of different vaccine approaches and their potential benefits.
Potential Future Directions and Breakthroughs
Future research in brain cancer vaccines is likely to focus on personalized approaches. This could involve tailoring vaccine formulations based on the specific genetic and molecular characteristics of individual patients’ tumors. Innovative delivery systems, such as nanoparticles or viral vectors, could potentially overcome the blood-brain barrier, enabling more effective vaccine delivery. Further exploration of immune checkpoint inhibitors, which can enhance the anti-tumor immune response, is also a promising area of investigation.
Another avenue of exploration is combining vaccines with other cancer therapies, such as chemotherapy or radiation, to enhance their efficacy and reduce side effects. This synergistic approach could potentially lead to improved clinical outcomes.
Current Progress in Animal Models
Ongoing research in animal models is evaluating various strategies for vaccine delivery and immune stimulation. Studies are exploring different vaccine formulations, including using tumor-associated antigens (TAAs) or oncolytic viruses. Researchers are also investigating methods to enhance immune cell infiltration into the tumor microenvironment. These studies are crucial for guiding the development of effective clinical trials in humans.
One promising approach involves using immune checkpoint inhibitors to boost the body’s own immune response against the tumor. Another approach is to employ oncolytic viruses, which infect and destroy tumor cells, in combination with vaccines to elicit a more robust anti-tumor response.
Patient Recruitment and Support
Recruiting participants for clinical trials, especially those involving complex diseases like brain cancer, requires careful consideration and a strong commitment to patient well-being. A successful trial relies not only on the scientific rigor of the design but also on the ability to attract and retain a diverse patient population while ensuring their comfort and safety throughout the process. Effective support systems and advocacy play crucial roles in this process.The recruitment process for brain cancer vaccine trials is multifaceted, often involving multiple institutions and patient outreach strategies.
Understanding the specific needs and concerns of potential participants is essential for building trust and encouraging participation.
Patient Recruitment Process
The recruitment process typically begins with identifying potential participants who meet the specific criteria Artikeld in the trial protocol. These criteria might include specific brain cancer types, stage of disease, and eligibility for specific treatments. Recruiting patients often involves collaborating with neuro-oncology specialists, hospitals, and cancer centers. A multi-faceted approach including online platforms, community outreach programs, and targeted advertising may be used to reach potential participants.
Support Systems for Participants
Comprehensive support systems are vital for participants throughout the trial process. This encompasses a range of services, including:
- Financial Assistance: Many trials offer financial support to cover travel expenses, lodging, and other out-of-pocket costs associated with participation. This is crucial as these expenses can be substantial, especially for patients living far from the trial site.
- Emotional Support: Mental health professionals and support groups provide emotional support and guidance to participants during the often-challenging experience of a clinical trial. Addressing the emotional burden of a brain cancer diagnosis is a crucial component of care.
- Transportation and Lodging: The trial site and access to transportation and accommodation are key considerations for participants, particularly those from geographically remote areas or facing mobility limitations. Trial coordinators may assist with travel arrangements and provide necessary support.
- Medical Monitoring: Continuous medical monitoring during the trial is essential for assessing treatment efficacy and adverse effects. This often involves regular checkups and communication with medical professionals.
These support systems help patients feel supported and involved in their care, increasing their willingness to participate.
Importance of Patient Advocacy
Patient advocacy plays a critical role in advancing brain cancer vaccine research. Patient advocates, often individuals or groups affected by the disease, bring invaluable insights into the challenges and needs of patients. They can help to identify gaps in research and advocate for improved access to treatments and support services.
Brain cancer vaccine clinical trials are showing promising early results, but a lot more research is needed. It’s inspiring to see organizations like the AADE, focusing on patient engagement and education, as exemplified in their commitment to the diabetes patient community, aade embracing diabetes patient community. This patient-centric approach could be a valuable model for future cancer vaccine trials, ensuring better patient outcomes and a more positive experience throughout the research process.
Examples of Successful Patient Advocacy Groups
Numerous patient advocacy groups have played a vital role in advancing research and improving the lives of individuals with brain cancer. These groups include:
- The Brain Tumor Foundation: This organization provides research funding, educational resources, and support for patients and families.
- The National Brain Tumor Society: This organization advocates for research, patient support, and awareness of brain tumors.
- The American Brain Tumor Association: This organization focuses on improving the lives of individuals affected by brain tumors through advocacy, research, and patient support.
Ethical Considerations in Patient Recruitment and Treatment
Ethical considerations are paramount in patient recruitment and treatment within clinical trials. Ensuring informed consent, fair selection processes, and equitable access to potential treatments is crucial.
- Informed Consent: Patients must be fully informed about the risks and benefits of the trial, including the potential side effects and any alternative treatments. This ensures that their participation is truly voluntary.
- Equity and Access: Recruitment efforts should strive for inclusivity and address potential disparities in access to trials based on socioeconomic status, geographic location, or other factors.
- Data Privacy and Security: Strict adherence to data privacy regulations is critical to protect the sensitive medical information of trial participants.
Public Health Impact and Policy
A successful brain cancer vaccine program could revolutionize cancer care, dramatically impacting public health. This impact would extend beyond individual patients, influencing healthcare systems and prompting crucial policy changes. Understanding the potential benefits and challenges is vital for shaping a future where brain cancer is less prevalent and more treatable.
Potential Public Health Impact
A highly effective brain cancer vaccine could significantly reduce the global burden of this disease. Lowering incidence rates would lead to fewer individuals suffering from this devastating illness, impacting families and communities positively. This would also translate to a reduced strain on healthcare resources, freeing up capacity for other health needs. Early detection and prevention through vaccination could alter the course of the disease, allowing for earlier intervention and improved patient outcomes.
Implications for Healthcare Systems
Implementing a brain cancer vaccination program would necessitate significant changes within healthcare systems. Increased demand for vaccine administration, storage, and monitoring would require substantial investment in infrastructure and personnel. Furthermore, the long-term management of vaccine safety and efficacy data will require ongoing monitoring and research. Integrating vaccination into existing healthcare programs and protocols will be crucial for seamless implementation and optimal reach.
The long-term cost-effectiveness of the vaccine will need to be meticulously assessed to ensure sustainable funding.
Examples of Successful Vaccination Programs
The success of vaccination programs for other cancers, such as the HPV vaccine for cervical cancer prevention, demonstrates the potential impact of preventative measures. The widespread adoption of vaccines for diseases like measles, mumps, and rubella showcases the effectiveness of preventive medicine in controlling infectious diseases. These examples highlight the positive impact of proactive public health interventions.
Importance of Public Awareness Campaigns
Public awareness campaigns will be critical in educating the public about the benefits and risks of brain cancer vaccines. These campaigns should address concerns and dispel myths surrounding vaccination. Transparency and open communication are essential for fostering public trust and encouraging participation in vaccination programs. Successful campaigns should provide clear and concise information about the vaccine’s efficacy, safety, and potential side effects.
Understanding the public’s perspective and tailoring messages accordingly is vital for effective communication.
Cost-Effectiveness of Brain Cancer Vaccine Programs
The cost-effectiveness of a brain cancer vaccine program will depend on various factors, including vaccine price, administration costs, and long-term healthcare savings. A comprehensive cost-benefit analysis, including potential reductions in healthcare costs due to reduced treatment needs, is essential to demonstrate the economic viability of such a program. Countries with robust healthcare systems and well-defined healthcare policies may be better positioned to implement such a program, considering factors like access to healthcare, population density, and economic resources.
Comparisons with other preventative healthcare measures and their cost-effectiveness are essential to guide resource allocation decisions.
Conclusion
The development of a brain cancer vaccine presents a beacon of hope for patients and researchers alike. While significant challenges remain, ongoing clinical trials are meticulously evaluating the safety and efficacy of these innovative approaches. The potential benefits, including improved survival rates and reduced recurrence, are compelling, but potential risks and side effects must be carefully considered. Ultimately, the success of these vaccines hinges on careful research, ethical considerations, and sustained public support.
This journey toward a potential cure demands continuous exploration and collaboration.