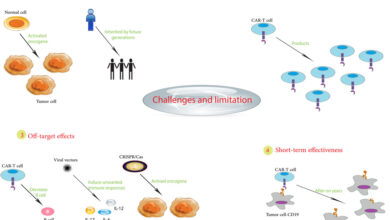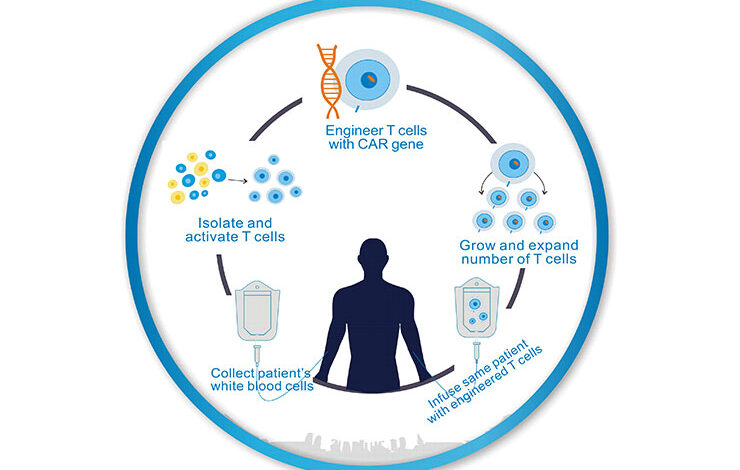
CAR T cancer treatment offers a revolutionary approach to battling various cancers. This treatment involves harnessing a patient’s own immune cells, modifying them to specifically target and destroy cancer cells. It’s a complex process with careful patient selection, specialized manufacturing, and rigorous monitoring, but the potential for long-term remission is significant. Understanding the nuances of this treatment, from its historical development to the latest research, is crucial for both patients and healthcare professionals.
This guide explores the key aspects of CAR T-cell therapy, from the fundamental principles of how it works to the challenges and potential outcomes. We’ll examine the patient selection process, the manufacturing and infusion techniques, the crucial role of monitoring side effects, and the long-term implications of this innovative treatment. Detailed tables provide a concise comparison of different therapies, patient assessments, manufacturing methods, and potential side effects, allowing for a comprehensive understanding of this complex treatment.
Introduction to CAR T-cell Therapy for Cancer
CAR T-cell therapy represents a revolutionary approach to cancer treatment. It harnesses the power of a patient’s own immune system to specifically target and destroy cancer cells. This personalized treatment approach holds immense promise for patients with certain types of blood cancers, offering a chance for remission and potentially a cure in some cases. The precision and targeted nature of this therapy distinguish it from traditional chemotherapy, minimizing harm to healthy cells.CAR T-cell therapy works by genetically modifying a patient’s own T cells, a type of white blood cell crucial for the immune response.
These modified T cells, called CAR T-cells, are then infused back into the patient. Crucially, the CAR T-cells are engineered to recognize and bind to specific proteins, called antigens, found on the surface of cancer cells. Once bound, the CAR T-cells trigger an immune response, leading to the destruction of the targeted cancer cells. This process is highly specific, allowing the immune system to effectively target cancer cells without harming healthy cells.
Mechanism of Action
The mechanism involves several key steps. First, T cells are extracted from the patient’s blood. Next, a special genetic material, called a chimeric antigen receptor (CAR), is inserted into these T cells. This CAR acts like a “smart bomb,” enabling the T cells to recognize and bind to specific antigens on the surface of cancer cells. Once the CAR T-cells bind to the target cancer cells, they release cytotoxic molecules and initiate a cascade of immune responses, leading to the death of the cancer cells.
This targeted approach helps to minimize side effects, as it primarily affects the cancerous cells.
Historical Context and Development
The development of CAR T-cell therapy builds upon decades of research in immunology and gene therapy. Early research focused on understanding the immune system’s ability to recognize and eliminate foreign invaders. Subsequent advancements in genetic engineering allowed for the precise modification of T cells, enabling the creation of CAR T-cells. This iterative process of discovery and innovation culminated in the first successful clinical trials and approvals of CAR T-cell therapies for specific blood cancers.
CAR T-cell therapy is a groundbreaking cancer treatment, but it’s not a one-size-fits-all solution. Meanwhile, Australia’s proactive approach to cervical cancer prevention, like using the HPV vaccine australia using hpv vaccine to eliminate cervical cancer , highlights the importance of preventative measures alongside innovative therapies. This highlights the need for a multifaceted approach to cancer treatment, combining targeted therapies like CAR T-cells with public health initiatives to improve outcomes.
Types of CAR T-cell Therapies
Various CAR T-cell therapies are currently available or under development, each targeting different antigens on cancer cells. These therapies vary in their target antigen, manufacturing process, and reported success rates. Understanding these distinctions is crucial for tailoring treatment to individual patient needs.
Comparison of CAR T-cell Therapies
| Therapy Name | Target Antigen | Manufacturing Method | Success Rate |
|---|---|---|---|
| Kymriah (tisagenlecleucel) | CD19 | Viral vector-based | High success rates in some patients with B-cell leukemia/lymphoma |
| Yescarta (axicabtagene ciloleucel) | CD19 | Viral vector-based | High success rates in some patients with B-cell leukemia/lymphoma |
| Other therapies in development | Various antigens (e.g., CD22, BCMA) | Viral vector-based or other methods | Varying success rates depending on the specific antigen and patient characteristics |
Note: Success rates can vary significantly depending on factors like the specific type of cancer, the patient’s overall health, and the specific CAR T-cell therapy used. Data is subject to ongoing research and clinical trials.
Patient Selection and Preparation
CAR T-cell therapy, while offering a potential cure for some cancers, is not suitable for everyone. Careful patient selection and rigorous pre-treatment preparation are crucial for maximizing the therapy’s effectiveness and minimizing risks. This involves a multi-faceted approach considering the patient’s overall health, the specific type of cancer, and the potential for complications.The decision to proceed with CAR T-cell therapy is a complex one, requiring a thorough evaluation of the patient’s medical history and current condition.
This process is intended to identify those most likely to benefit from the treatment while mitigating the potential side effects. Each patient’s situation is unique, and personalized treatment plans are developed to address individual needs.
Patient Selection Criteria
Patients considered for CAR T-cell therapy typically have relapsed or refractory cancers that have not responded to other treatments. The specific criteria vary based on the type of cancer, but generally include factors like the patient’s age, overall health, and the stage and type of the disease. These factors help determine the likelihood of a successful outcome and the potential for adverse reactions.
Pre-Treatment Procedures and Assessments
A series of assessments and procedures are necessary to prepare a patient for CAR T-cell infusion. These ensure the patient is in the best possible condition to tolerate the treatment and maximize its effectiveness.
- Baseline Assessments: Comprehensive baseline assessments, including complete blood counts, organ function tests (liver and kidney), and a thorough medical history, are critical to evaluating the patient’s health status before treatment. These initial assessments serve as a benchmark for monitoring the patient’s response and identifying any potential complications during the treatment process.
- Specific Cancer Evaluation: The specific type and stage of the cancer are evaluated to determine the suitability of CAR T-cell therapy. This includes factors like the presence of any other medical conditions, or if the cancer has spread to other parts of the body.
- Informed Consent: A detailed explanation of the risks and benefits of CAR T-cell therapy, including potential side effects, is crucial. Patients must provide informed consent before proceeding, ensuring they fully understand the treatment plan.
Potential Risks and Complications
CAR T-cell therapy, while potentially life-saving, carries significant risks. These include cytokine release syndrome (CRS), a potentially life-threatening inflammatory response, and neurologic complications. Careful monitoring and prompt intervention are essential to manage these risks.
Examples of Patient Demographics and Suitability
A young adult with acute lymphoblastic leukemia (ALL) who has relapsed after standard treatments might be a suitable candidate for CAR T-cell therapy. Similarly, an older adult with a specific type of lymphoma that has not responded to other treatments could also be considered. The suitability depends on a variety of factors, including the specific type and stage of the cancer, the patient’s overall health, and the potential risks and benefits.
CAR T-cell therapy is making incredible strides in cancer treatment, offering hope for patients with various blood cancers. While advancements like these are truly inspiring, it’s important to remember that other health concerns are still present. For example, the recent news that Zika won’t travel far in the USA ( zika wont travel far in usa ) provides a sense of relief and focus on the localized nature of certain health threats, allowing medical professionals to concentrate on innovative treatments like CAR T-cell therapy without additional distractions.
This ultimately benefits the continued development of groundbreaking cancer treatments.
Pre-Treatment Assessments
| Assessment Type | Purpose | Normal Range | Abnormal Range |
|---|---|---|---|
| Complete Blood Count (CBC) | Evaluates blood cell counts (red blood cells, white blood cells, platelets) | Within established reference ranges specific to the laboratory | Low red blood cell count, low white blood cell count, low platelet count |
| Liver Function Tests (LFTs) | Assesses liver function | Within established reference ranges specific to the laboratory | Elevated liver enzymes, suggesting liver dysfunction |
| Kidney Function Tests (KFTs) | Evaluates kidney function | Within established reference ranges specific to the laboratory | Elevated creatinine or blood urea nitrogen (BUN), indicating kidney impairment |
| Coagulation Profile | Evaluates blood clotting factors | Within established reference ranges specific to the laboratory | Abnormal clotting times, suggesting bleeding or clotting disorders |
| Electrolyte Panel | Evaluates levels of essential electrolytes in the blood | Within established reference ranges specific to the laboratory | Abnormal electrolyte levels, indicating potential imbalances |
CAR T-cell Manufacturing and Infusion: Car T Cancer Treatment
CAR T-cell therapy represents a groundbreaking approach to cancer treatment, harnessing the power of a patient’s own immune system to target and destroy cancerous cells. A critical aspect of this therapy is the meticulous process of manufacturing and infusing these modified T-cells. This involves a complex series of steps, each carefully orchestrated to maximize the effectiveness and minimize the risks associated with the treatment.
Collecting Patient T-cells
The journey begins with the collection of T-cells, a type of white blood cell crucial to the immune response. These cells are vital components of the body’s natural defense mechanisms. Apheresis, a process involving the separation of blood components, is commonly employed to isolate T-cells from the patient’s bloodstream. This procedure, while often performed under general anesthesia, is generally well-tolerated and involves the controlled withdrawal of blood, separation of the desired T-cells, and return of the remaining blood components to the patient.
CAR T-cell therapy is revolutionizing cancer treatment, offering a powerful approach to fight various forms of the disease. However, while this innovative treatment holds immense promise, it’s important to remember that treating rabies symptoms with rabies itself is unfortunately not a viable or safe approach, as detailed in this article can you treat rabies symptoms with rabies.
Ultimately, CAR T-cell therapy’s precision and targeted nature make it a truly exciting advancement in the fight against cancer.
The collected T-cells are then carefully prepared for the next stage of modification.
Modifying T-cells to Target Cancer
Once isolated, the T-cells undergo a crucial modification: equipping them with a chimeric antigen receptor (CAR). This engineered receptor acts as a “homing device,” guiding the T-cells to specifically recognize and attack cancer cells. The process typically involves genetic engineering, introducing the CAR gene into the T-cells using viral vectors. This new gene allows the T-cells to identify and destroy cancer cells with greater efficiency and precision.
Infusing Modified T-cells
The modified T-cells, now armed with the CAR, are then infused back into the patient’s bloodstream. This infusion is usually performed intravenously, allowing the T-cells to circulate throughout the body and locate cancerous cells. The patient’s body then recognizes and utilizes these modified T-cells to combat the cancer, effectively acting as a personalized cancer-fighting force.
Viral Vectors in CAR T-cell Production
Various viral vectors are employed in the genetic modification process. These vectors act as delivery vehicles for the CAR gene into the T-cells. Commonly used vectors include lentiviruses, retroviruses, and adeno-associated viruses. Each vector has unique characteristics, impacting the efficiency of gene transfer and the long-term safety of the therapy. The choice of vector often depends on the specific needs of the patient and the type of cancer being treated.
Comparison of T-cell Isolation, Modification, and Infusion Methods
| Method | Description | Advantages | Disadvantages |
|---|---|---|---|
| Apheresis | Blood is withdrawn, components separated, and the T-cells collected. | Relatively safe and effective method for isolating T-cells. | Requires specialized equipment and trained personnel. |
| Lentiviral Vector | Genetically modifies T-cells by integrating the CAR gene into the T-cell DNA. | High efficiency of gene transfer. | Potential for insertional mutagenesis, where the vector insertion disrupts another gene. |
| Retroviral Vector | Similar to lentiviral, but integration is limited to dividing cells. | Less risk of insertional mutagenesis compared to lentiviral vectors. | Limited to dividing cells, making it less efficient for some cell types. |
| Adeno-Associated Viral Vector (AAV) | Delivers the CAR gene without integrating into the host DNA. | Minimal risk of insertional mutagenesis. | Lower gene transfer efficiency compared to lentiviral vectors. |
| Intravenous Infusion | Administering the modified T-cells into the patient’s bloodstream. | Allows for systemic distribution of T-cells throughout the body. | Potential for side effects, such as cytokine release syndrome. |
Monitoring and Management of Side Effects
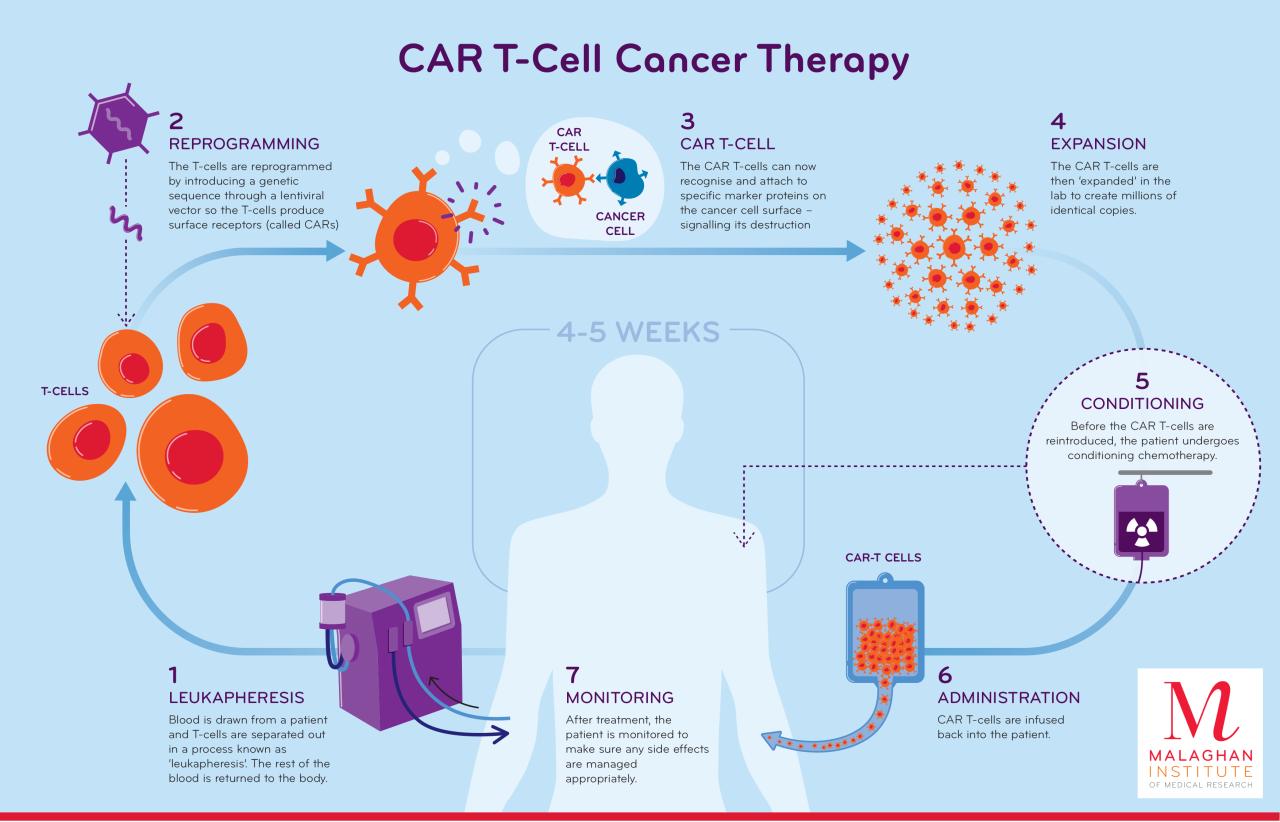
CAR T-cell therapy, while a powerful tool in the fight against cancer, can unfortunately come with a range of potential side effects. Understanding these side effects, how they’re monitored, and the strategies used to manage them is crucial for both patients and healthcare providers. Proper management is essential for ensuring patient comfort and maximizing the effectiveness of the therapy.
Potential Side Effects of CAR T-Cell Therapy
CAR T-cell therapy, while revolutionary, can trigger a range of adverse effects. These effects can vary in severity and duration, impacting different individuals in distinct ways. Recognizing the potential spectrum of side effects allows for proactive monitoring and management.
Methods Used to Monitor Side Effects
Monitoring patients undergoing CAR T-cell therapy is a continuous process, encompassing various methods. This meticulous approach ensures prompt identification of emerging side effects and allows for timely intervention. Frequent blood tests, physical examinations, and symptom assessments are crucial components of this comprehensive monitoring strategy. Vital signs, including temperature, heart rate, and blood pressure, are routinely checked to detect any fluctuations that might indicate an emerging issue.
Common Complications and Management Strategies
Cytokine release syndrome (CRS) and neurologic toxicity are two of the most common and potentially severe complications associated with CAR T-cell therapy. CRS is characterized by a systemic inflammatory response, while neurologic toxicity can manifest as various neurological symptoms.
| Side Effect | Description | Severity | Management Strategy |
|---|---|---|---|
| Cytokine Release Syndrome (CRS) | A systemic inflammatory response, characterized by fever, chills, nausea, and low blood pressure. | Mild to Severe | Supportive care, including fluids, antipyretics, and medications to manage symptoms. High-dose steroids are often used to reduce inflammation. |
| Neurologic Toxicity | A range of neurological symptoms, including confusion, seizures, and headaches. | Mild to Severe | Supportive care, including monitoring for seizures, and medications to manage symptoms. Corticosteroids and other medications may be used. |
| Encephalopathy | A severe brain dysfunction, characterized by changes in mental status, seizures, and coma. | Severe | Intensive care unit (ICU) monitoring, supportive care, and medications to manage symptoms. |
| Cardiovascular issues | Problems affecting the heart, such as arrhythmias and heart failure. | Mild to Severe | Monitoring heart rate and rhythm, and appropriate medications to manage symptoms. |
| Infections | Increased susceptibility to infections due to weakened immune system. | Mild to Severe | Prophylactic antibiotics and vigilant monitoring for signs of infection. |
Managing Side Effects
Effective management of side effects is crucial for patient well-being and treatment success. Treatment plans should incorporate individualized approaches tailored to each patient’s unique needs and response to the therapy. Early intervention and appropriate supportive care are key components of successful management. The use of medications to mitigate specific symptoms and address underlying causes is vital. Close monitoring by healthcare professionals is essential to identify and address any potential issues.
Long-Term Outcomes and Success Rates
CAR T-cell therapy, while revolutionary in cancer treatment, is not a one-and-done procedure. Understanding the long-term effects and success rates is crucial for both patients and healthcare professionals. This involves examining not just initial remission, but also the potential for relapse and the impact on overall quality of life. Long-term follow-up is essential to fully assess the efficacy of this complex treatment.
Factors Influencing Long-Term Outcomes
Several factors play a significant role in determining the long-term success of CAR T-cell therapy. Patient-specific characteristics, such as the type and stage of cancer, overall health, and immune system response, all influence the treatment’s efficacy. The specific CAR T-cell product used, the manufacturing process, and the administration method are also key factors. Furthermore, adherence to post-treatment monitoring and management protocols directly correlates with positive long-term outcomes.
Careful consideration of these elements helps in predicting potential challenges and improving patient care.
Overall Success Rates
CAR T-cell therapy demonstrates promising success rates for various cancers, particularly in blood cancers like leukemia and lymphoma. However, success rates vary considerably depending on the specific cancer type and individual patient characteristics. Early responses are often encouraging, but long-term remission and survival rates remain a subject of ongoing research and clinical trials. The complexity of the treatment necessitates continuous monitoring and adaptation of treatment plans to address evolving patient needs.
Long-Term Survival and Complications
Predicting long-term survival is complex. While some patients experience long-term remission, others may face relapses. The duration and severity of treatment-related side effects also vary. Recognizing these variations is crucial for tailoring individual care plans and improving overall patient outcomes.
Summary of Long-Term Outcomes
| Cancer Type | Success Rate (approximate) | Long-Term Survival Rate (approximate) | Potential Complications |
|---|---|---|---|
| Acute Lymphoblastic Leukemia (ALL) | 60-80% | 50-70% (depending on risk factors) | Cytokine release syndrome (CRS), neurotoxicity, immune effector cell-associated neurotoxicity syndrome (ICANS), infections |
| B-cell Lymphoma | 50-70% | 40-60% (depending on lymphoma subtype) | CRS, neurotoxicity, graft-versus-host disease (GVHD), infections |
| Multiple Myeloma | 30-50% | 20-40% (variable response) | CRS, neurotoxicity, infections, renal impairment |
| Chronic Lymphocytic Leukemia (CLL) | 40-60% | 30-50% (depending on CLL subtype) | CRS, neurotoxicity, infections, progressive disease |
Note: Success rates and survival data are approximate and can vary significantly based on individual patient factors, treatment protocols, and disease characteristics. This table is for illustrative purposes only and should not be used for self-diagnosis or treatment decisions. Consult with a qualified healthcare professional for personalized guidance.
Research and Future Directions
CAR T-cell therapy has shown remarkable promise in treating certain cancers, but significant challenges and limitations remain. Ongoing research is crucial for expanding its application, enhancing efficacy, and minimizing side effects. This exploration delves into current research areas, potential future innovations, and the hurdles that need to be overcome.
Current Research Areas
Researchers are actively investigating various avenues to improve CAR T-cell therapy. One key area focuses on engineering more potent and durable CAR T-cell products. This includes optimizing CAR design for enhanced target recognition and reduced off-target effects. Other areas of investigation include improving manufacturing processes to ensure consistent product quality and scalability.
Potential Future Developments
Several innovative approaches hold promise for advancing CAR T-cell therapy. One area of interest is the development of “universal” CAR T-cells capable of targeting a broader range of cancers. This would significantly expand the treatment options available. Another area involves the combination of CAR T-cell therapy with other cancer treatments, such as chemotherapy or immunotherapy, to achieve synergistic effects.
The use of advanced bioengineering techniques to improve CAR T-cell persistence and efficacy is also under investigation.
Challenges and Limitations
Despite its success, CAR T-cell therapy faces challenges. One significant hurdle is the high cost of manufacturing and administering the therapy. Furthermore, the development of severe side effects, known as cytokine release syndrome (CRS) and neurotoxicity, remains a concern. Another limitation lies in the limited efficacy against certain cancers and the need for personalized approaches. The logistical and administrative complexities associated with manufacturing, testing, and delivering CAR T-cell therapies also present a hurdle.
Areas Requiring Further Research, Car t cancer treatment
Several areas demand further investigation to address the limitations of current CAR T-cell therapies. One area is developing strategies to mitigate the severe side effects associated with treatment. Another priority is the exploration of innovative approaches for broader cancer targeting. Improving the manufacturing process for cost-effectiveness and scalability is also essential. The development of predictive models for patient selection and treatment response would further refine treatment strategies.
Table of Potential Future Directions
| Research Area | Goal | Method | Potential Outcomes |
|---|---|---|---|
| Enhanced CAR Design | Improve target specificity and reduce off-target effects | Engineering CARs with novel modifications (e.g., improved binding domains, enhanced signaling pathways) | Increased efficacy, reduced side effects, expanded treatment options for more cancers |
| Improved Manufacturing Processes | Increase consistency and scalability of CAR T-cell production | Development of more efficient and cost-effective manufacturing techniques, automation, and optimized cell culture conditions | Lower production costs, more accessible therapy, faster production time |
| Combination Therapies | Enhance efficacy and reduce side effects by combining CAR T-cells with other treatments | Clinical trials combining CAR T-cell therapy with chemotherapy, radiation, or other immunotherapies | Synergistic effects, improved outcomes, potentially treating a wider range of cancers |
| Targeting Multiple Cancers | Develop universal CAR T-cells targeting a wider range of cancer antigens | Engineering CARs targeting conserved or common antigens across various cancers | Expansion of treatment options for previously untreatable cancers, improved patient outcomes, reduced need for extensive testing |
Conclusion

In conclusion, CAR T-cell therapy represents a groundbreaking advancement in cancer treatment. While the process is intricate, with careful consideration of patient selection, manufacturing, and ongoing monitoring, the potential for long-term remission is substantial. This treatment is still under active research and development, and future innovations promise even greater success. The journey to personalize and optimize CAR T-cell therapy is ongoing, promising a future with improved outcomes for cancer patients.