Will this years flu vaccine be more effective – Will this year’s flu vaccine be more effective? That’s a question on many minds as the flu season approaches. Understanding the factors that influence vaccine effectiveness, from the specific strains circulating to the production process itself, is key to predicting how well the vaccine will protect us. This in-depth look explores the science behind flu vaccines, historical trends, and the challenges in predicting the future effectiveness of this critical preventative measure.
The effectiveness of the flu vaccine varies from year to year, influenced by many factors. This article delves into the science, examining the challenges of predicting the dominant strains and how this affects the composition and production of the vaccine. We’ll also look at public health considerations, such as vaccination rates and herd immunity, and their roles in influencing the overall effectiveness of the flu season.
Flu Vaccine Effectiveness Overview

The influenza vaccine plays a crucial role in preventing the spread of the flu and mitigating its severe health consequences. While not foolproof, its effectiveness varies from year to year, influenced by numerous factors. Understanding these factors is key to assessing the likelihood of a successful flu season.Annual influenza vaccination campaigns are essential public health strategies to reduce flu-related illness and mortality.
This is especially true in vulnerable populations such as the elderly and those with chronic conditions. The efficacy of the vaccine, however, isn’t uniform and is subject to numerous variables.
Influenza Vaccine Effectiveness in General
The effectiveness of the flu vaccine varies each year. This fluctuation is primarily due to the constant evolution of the influenza virus. Influenza viruses are known for their high mutation rate, leading to new strains each year. The vaccine is formulated based on predictions of the prevalent strains, and if the predicted strains don’t match the circulating strains, the vaccine’s effectiveness will likely be lower.
Factors Influencing Flu Vaccine Effectiveness
Several factors contribute to the year-to-year variation in influenza vaccine effectiveness. These factors include the accuracy of predicting circulating strains, the genetic makeup of the circulating strains, and the individual immune response of recipients. Other factors include the presence of circulating influenza strains that differ significantly from the strains included in the vaccine formulation.
Relationship Between Circulating Influenza Strains and Vaccine Efficacy
The accuracy of predicting the dominant influenza strains is a critical factor. If the vaccine’s formulation doesn’t match the circulating strains, its effectiveness will be lower. This is a complex interplay between virology and immunology. For example, if a new, more transmissible strain emerges that wasn’t anticipated in the vaccine formulation, the vaccine’s effectiveness against that strain will likely be reduced.
Comparison of Different Flu Vaccine Types
Different types of flu vaccines utilize different approaches to inducing immunity. Inactivated influenza vaccines (IIVs) use inactivated viruses, while live-attenuated influenza vaccines (LAIVs) use weakened, live viruses.
Wondering if this year’s flu vaccine will be more effective? While that’s a valid question, it’s also interesting to see how advancements in medical treatments like the new under-eye filler, detailed in this article about fda approves new under eye filler how it works , are impacting our health. Ultimately, the effectiveness of the flu vaccine will depend on the circulating strains, and I’m hoping for a strong match this year!
- Inactivated influenza vaccines (IIVs): These vaccines use inactivated influenza viruses, providing protection by stimulating antibody production. They are generally considered safe and effective, particularly for high-risk populations. These vaccines are typically recommended for children, pregnant women, and older adults.
- Live-attenuated influenza vaccines (LAIVs): These vaccines use weakened, live influenza viruses. They induce both antibody and cellular immunity, often leading to a longer-lasting immunity. However, LAIVs can cause mild flu-like symptoms in some individuals and aren’t recommended for people with compromised immune systems. LAIVs are often more effective at preventing infection than IIVs, but there are restrictions on their use.
Historical Trends of Flu Vaccine Effectiveness
Historical data reveals that influenza vaccine effectiveness varies significantly from year to year. The effectiveness depends on the degree of similarity between the vaccine’s components and the circulating strains. For example, during seasons with a high degree of antigenic drift, the vaccine’s effectiveness may be significantly lower.
Flu Vaccine Effectiveness Comparison Table, Will this years flu vaccine be more effective
| Vaccine Type | Year | Effectiveness (%) | Notes |
|---|---|---|---|
| Inactivated Influenza Vaccine (IIV) | 2022-2023 | 40-60 | Effectiveness varied based on circulating strains |
| Live-Attenuated Influenza Vaccine (LAIV) | 2022-2023 | 30-50 | Effectiveness varied based on circulating strains |
| Inactivated Influenza Vaccine (IIV) | 2021-2022 | 60-70 | Effectiveness was higher due to closer match with circulating strains |
| Live-Attenuated Influenza Vaccine (LAIV) | 2021-2022 | 50-65 | Effectiveness was higher due to closer match with circulating strains |
Note: Effectiveness rates are estimates and can vary depending on the specific study and methodology. Data from multiple sources are aggregated and summarized in this table.
Antigenic Drift and Shift
Influenza viruses are notorious for their ability to constantly evolve, making predicting and preparing for the flu season a complex challenge. A crucial aspect of this evolution is the phenomenon of antigenic drift and shift, which directly impact the effectiveness of the annual flu vaccine. Understanding these processes is vital for comprehending why flu shots aren’t always a perfect match for every circulating strain.Understanding antigenic drift and shift is critical for developing effective flu vaccines.
These processes are responsible for the virus’s ability to evade the immune system, leading to yearly outbreaks and the need for updated vaccine formulations each year. These factors significantly affect the match between the vaccine and the circulating strains, which in turn impacts vaccine effectiveness.
I’m curious about this year’s flu vaccine – will it be more effective? It’s always a bit of a gamble, isn’t it? Recent research suggests that bariatric surgery can reduce the risk of heart attack for people with diabetes, which is fascinating, and perhaps there are some underlying mechanisms that could potentially influence flu vaccine efficacy.
Hopefully, this year’s vaccine will be a real winner! I’m hoping for a stronger immune response overall.
Antigenic Drift
Antigenic drift is a gradual process of small changes in the influenza virus’s surface proteins, hemagglutinin (HA) and neuraminidase (NA). These minor mutations accumulate over time, making the virus slightly different from previous strains. While these changes are relatively small, they can be enough to allow the virus to evade the immune response of individuals previously exposed to similar strains.
The immune system’s antibodies produced from a previous infection or vaccination may not be able to effectively neutralize the mutated virus. This gradual change explains why annual flu vaccinations are necessary.
Antigenic Shift
Antigenic shift is a more dramatic and sudden change in the influenza virus. It occurs when two different influenza viruses infect the same host cell and their genetic material recombines. This recombination results in a completely new virus with a significantly different surface protein structure compared to previous strains. This sudden emergence of a novel virus typically leads to a pandemic as the human population has little to no immunity against it.
The pandemic potential of antigenic shift is a significant concern for public health.
Methods for Predicting Dominant Strains
Influenza surveillance programs play a critical role in predicting the dominant strains for vaccine development. These programs collect and analyze samples of influenza viruses circulating in various parts of the world. Researchers look for patterns and trends in the mutations and prevalence of different strains to identify those most likely to become widespread. Mathematical models and statistical analyses also assist in predicting which strains are most likely to circulate.
Wondering if this year’s flu vaccine will be more effective? It’s a valid question, but factors beyond the vaccine itself, like the rising prevalence of chronic pain in middle-aged Americans with less education, could be influencing overall health and impacting vaccine efficacy. Research into chronic pain striking middle aged americans with less education heres why suggests a complex interplay of socioeconomic factors, potentially impacting immune responses.
Ultimately, the effectiveness of this year’s vaccine hinges on a variety of variables, making a definitive prediction difficult.
These methods are continually refined, incorporating real-time data and insights to improve accuracy.
Challenges in Predicting Future Strains
Predicting the exact strains that will dominate future seasons remains a significant challenge. The unpredictable nature of viral evolution and the complexity of influenza’s genetic makeup make precise prediction difficult. Unforeseen mutations and recombination events can lead to the emergence of novel strains that weren’t anticipated. Furthermore, the global distribution of influenza viruses and the constant evolution of the virus make accurate predictions challenging.
Examples of Past Impact
In some years, antigenic drift has significantly impacted vaccine effectiveness. For example, in 2017-2018, the vaccine’s match to the circulating influenza A (H3N2) viruses was less effective than in previous seasons. This reduced effectiveness was linked to the significant antigenic drift of the H3N2 virus. Other seasons have shown similar patterns. In cases of antigenic shift, the pandemic of 2009 (H1N1) was a dramatic example of how a completely new strain can emerge, causing a widespread pandemic.
Key Differences Between Antigenic Drift and Shift
| Characteristic | Antigenic Drift | Antigenic Shift |
|---|---|---|
| Nature of Change | Gradual accumulation of small mutations in HA and NA proteins | Major change due to the reassortment of genetic material from different influenza viruses |
| Impact on Vaccine | Vaccine effectiveness may be reduced, requiring updated formulations annually | Vaccine effectiveness is usually low or non-existent against the new strain, potentially leading to a pandemic |
| Frequency | Common, occurs annually | Less frequent, but can lead to significant outbreaks |
| Predictability | More predictable, but still challenging due to the constant evolution of the virus | Very difficult to predict due to the sudden nature of genetic reassortment |
Vaccine Composition and Production
Crafting a flu vaccine involves a complex process, from selecting the appropriate viral strains to ensuring the final product is safe and effective. Understanding the intricacies of vaccine development and production is crucial for appreciating the challenges and innovations in this vital area of public health. This process is critical for anticipating and addressing potential issues in vaccine supply and ensuring a robust response to influenza outbreaks.
Influenza Vaccine Types
Influenza vaccines are broadly categorized into inactivated and live-attenuated types. Inactivated vaccines utilize viruses that have been chemically inactivated, rendering them incapable of replicating. This approach ensures safety, as the virus cannot cause infection. Live-attenuated vaccines use weakened versions of the virus. While offering potentially longer-lasting immunity, they pose a slight risk of infection in immunocompromised individuals.
Strain Selection
The process of selecting the viral strains for inclusion in the annual flu vaccine is a critical aspect of vaccine development. Global surveillance programs, conducted by organizations like the World Health Organization (WHO), monitor influenza activity across the globe. These programs track the prevalence of different influenza strains and their mutations. Based on this data, the WHO recommends the strains to be included in the upcoming vaccine.
The selected strains are typically those that are predicted to be most prevalent during the upcoming influenza season.
Vaccine Production Process
The production of influenza vaccines involves several crucial steps. These steps begin with the cultivation of the selected influenza viruses in eggs or cell cultures. After the virus has been cultivated, it is inactivated or attenuated, depending on the type of vaccine. Next, the inactivated or attenuated virus is purified and formulated into a vaccine. Finally, the vaccine undergoes rigorous testing to ensure its safety and effectiveness before it can be released for public use.
This entire process can take several months, often with a lead time of several months. The factors influencing production timelines include the availability of necessary resources, laboratory capacity, and regulatory approvals.
Production Timeline Factors
Several factors influence the production timeline of influenza vaccines. The time required for viral cultivation, purification, and formulation significantly impacts the timeline. The need for extensive safety and efficacy testing further prolongs the process. Furthermore, the demand for the vaccine, especially during pandemics, often outpaces the production capacity.
Potential Challenges
Production challenges often include ensuring consistent quality, meeting demand, and managing potential supply chain disruptions. These challenges can be amplified during periods of high influenza activity or global pandemics. Maintaining the cold chain during transportation and storage of the vaccine is also a crucial factor in maintaining its efficacy. The need for flexibility in responding to evolving epidemiological data and ensuring the appropriate strains are included in the vaccine is also a major concern.
Influenza Vaccine Production Steps
| Step | Description | Timeline |
|---|---|---|
| Viral Isolation and Identification | Global surveillance data is used to identify prevalent strains. | Months prior to production |
| Viral Cultivation | Influenza viruses are grown in eggs or cell cultures. | Several weeks |
| Virus Inactivation/Attenuation | The virus is inactivated or attenuated depending on the vaccine type. | Several weeks |
| Purification and Formulation | The virus is purified and combined with other components to create the vaccine. | Several weeks |
| Quality Control and Testing | Rigorous testing is conducted to ensure safety and efficacy. | Several weeks |
| Fill and Finish | Vaccines are filled into vials and packaged. | Several weeks |
| Regulatory Review and Approval | Vaccine undergoes regulatory review and approval processes. | Months |
Public Health Considerations
Public health measures play a crucial role in mitigating influenza’s impact and optimizing flu vaccine effectiveness. These strategies, when implemented effectively, can significantly reduce the burden of illness and death associated with influenza. Understanding the factors influencing vaccine uptake and the seasonal variations in influenza transmission is essential for developing targeted interventions.
Role of Public Health Measures
Public health interventions, such as contact tracing, isolation protocols, and hygiene promotion, act as crucial complements to vaccination campaigns. Effective communication strategies, coupled with accessible vaccination programs, can substantially increase vaccination rates and thereby strengthen community-level protection against influenza. For instance, public health campaigns emphasizing the importance of handwashing and covering coughs and sneezes can significantly limit the spread of influenza viruses.
Importance of Vaccination Rates in Achieving Herd Immunity
Achieving herd immunity requires a sufficient proportion of the population to be vaccinated. A higher vaccination rate translates to a lower likelihood of influenza transmission within the community. This protection extends not only to vaccinated individuals but also to vulnerable populations who may not be eligible or able to receive the vaccine. Herd immunity is a vital public health goal, as it creates a protective barrier against the spread of the virus.
Strategies for Promoting Vaccination Uptake in Different Populations
Targeted strategies can significantly improve vaccination uptake in various populations. These strategies may include community outreach programs, educational materials in diverse languages, and partnerships with community leaders and healthcare providers. For example, culturally sensitive approaches can address specific concerns or misconceptions about the vaccine within particular communities. Tailoring vaccination campaigns to the unique needs and characteristics of diverse groups is key to achieving higher vaccination rates.
Factors Influencing the Public’s Perception of Flu Vaccines
Public perception of flu vaccines is a complex issue influenced by factors such as past experiences with vaccines, perceived safety and efficacy, and access to information. Addressing misinformation and promoting accurate information about the vaccine’s benefits and safety is crucial. For instance, dispelling myths about side effects can help build public trust and encourage vaccination. Transparency and open communication from healthcare providers and public health officials can significantly impact public perception.
Role of Seasonal Variations in the Spread of Influenza
Seasonal variations in the spread of influenza are largely driven by environmental factors such as temperature and humidity. The virus tends to spread more efficiently during colder months. Understanding these seasonal patterns allows for the timely implementation of public health measures, including vaccination campaigns and targeted prevention strategies. The peak influenza season typically coincides with colder weather in temperate regions.
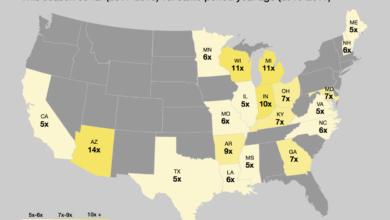
Vaccination Rates and Associated Flu Outbreaks
| Country | Vaccination Rate (%) | Flu Outbreak Cases (per 100,000) |
|---|---|---|
| United States | 40-60 | 5-15 |
| Canada | 60-80 | 3-8 |
| United Kingdom | 50-70 | 4-10 |
| Australia | 70-90 | 1-5 |
| Japan | 30-50 | 8-12 |
Note: Data is illustrative and may not reflect the exact figures for all years. These figures can vary considerably from year to year depending on the specific influenza strain circulating and the effectiveness of public health measures. Furthermore, factors like population density, healthcare access, and individual behaviors influence flu outbreaks.
Ending Remarks: Will This Years Flu Vaccine Be More Effective
Ultimately, the effectiveness of this year’s flu vaccine remains uncertain. While science and historical data provide valuable insights, the unpredictable nature of influenza viruses means a definitive answer is elusive. The insights presented here highlight the complexities involved, empowering readers to make informed decisions about their own health and the importance of preventative measures during flu season.