Who can participate in a clinical trial? This isn’t just about picking volunteers; it’s about understanding the specific rules and guidelines that ensure a fair and successful trial. Eligibility criteria vary significantly depending on the type of trial, from early-stage research to large-scale studies. Factors like age, health conditions, and lifestyle choices play a crucial role in determining who’s suitable to participate.
Let’s dive into the world of clinical trials and explore the complexities of inclusion and exclusion criteria, recruitment strategies, and ethical considerations that underpin this critical process.
Understanding the specific eligibility criteria is essential for anyone considering participating in a clinical trial. These criteria are carefully designed to ensure the trial’s participants are appropriate for the research being conducted. Different trials have different criteria based on factors like the type of disease being studied, the stage of the disease, and the treatment being tested. This is crucial to ensure the trial’s results are valid and reliable.
Eligibility Criteria
Clinical trials are crucial for advancing medical knowledge and improving patient care. A key component of successful trials is the selection of participants who are most likely to benefit from the treatment and who can contribute meaningful data. This selection process is governed by specific eligibility criteria. Understanding these criteria is essential for both potential participants and researchers.
Factors Influencing Eligibility
Eligibility criteria for clinical trials are multifaceted and consider various factors. These factors encompass a range of aspects, from demographics to medical history and lifestyle choices. Each clinical trial has unique eligibility criteria tailored to its specific research question and the characteristics of the intervention being studied.
Eligibility Criteria Across Trial Phases
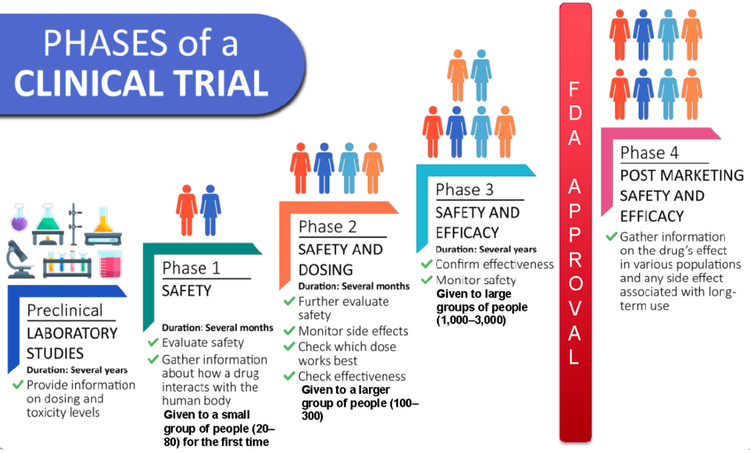
Clinical trials are typically categorized into phases, each with distinct goals and participant characteristics. Phase 1 trials primarily focus on safety, while later phases, such as Phase 3, assess efficacy and compare the new treatment to existing standards of care. This difference in focus directly impacts the eligibility criteria. Phase 1 trials often have more stringent eligibility requirements, aiming for a specific patient population that might experience the treatment in a safe and manageable way.
Cardiovascular Disease Trial Eligibility Criteria, Who can participate in a clinical trial
Cardiovascular disease trials often target individuals with specific conditions and risk factors. The following table Artikels some common eligibility criteria for a hypothetical cardiovascular disease clinical trial.
| Eligibility Criteria | Specific Examples of Conditions | Explanation |
|---|---|---|
| Age | 45-75 years | The trial focuses on a middle-aged and older population who are at higher risk of cardiovascular issues. |
| Diagnosis of Heart Failure | New-onset heart failure within the last 6 months | Participants must have recently developed heart failure to ensure the trial accurately measures the effects of the treatment on a newly diagnosed patient population. |
| Ejection Fraction | 35-55% | Participants must have a specific ejection fraction (percentage of blood pumped out of the heart with each beat) to ensure homogeneity within the trial group. |
| Stable Medical History | No recent hospitalizations or significant changes in medications | Participants with stable conditions are less likely to confound the results of the trial. |
| Lifestyle Factors | Non-smokers, healthy diet, regular exercise | Lifestyle factors influence cardiovascular health and are important to consider to maintain consistency across participants. |
Influence of Age, Gender, Medical History, and Lifestyle Factors
Age, gender, pre-existing medical conditions, and lifestyle choices are critical factors in determining trial eligibility. Age often influences the types of conditions being studied and the potential risks associated with the treatment. Gender-based differences in disease prevalence and response to treatment can also impact eligibility criteria. Individuals with pre-existing conditions might be excluded if those conditions could confound the results or pose a risk.
Lifestyle factors, such as smoking, diet, and exercise, can affect the treatment’s efficacy and safety, influencing participant selection.
Table of Eligibility Criteria
This table provides examples of various eligibility criteria, the conditions they relate to, and their explanations.
| Eligibility Criteria | Specific Examples of Conditions | Explanation |
|---|---|---|
| Specific Cancer Type | Stage II-III breast cancer | The trial focuses on patients with a specific stage of breast cancer. |
| Medication Use | Currently taking a specific type of anti-depressant | Participants who are taking a particular medication might be excluded or included based on the need to control for confounding factors. |
| Genetic predisposition | Known BRCA1 mutation carrier | This factor might be considered for specific genetic-based treatments. |
| Body Mass Index (BMI) | 18-25 kg/m² | Participants must have a specific BMI range to ensure homogeneity in the trial. |
Inclusion and Exclusion Criteria
Clinical trials are meticulously designed experiments aimed at evaluating the efficacy and safety of new treatments or interventions. To ensure the validity and reliability of the results, researchers establish specific criteria for participant selection. This crucial process, encompassing inclusion and exclusion criteria, defines the characteristics of individuals eligible to participate in the trial.Understanding these criteria is essential for participants to make informed decisions about their involvement and for researchers to ensure that the trial population accurately reflects the intended target group.
It is vital to recognize the importance of these criteria for the accuracy and generalizability of the findings.
Importance of Inclusion and Exclusion Criteria
Inclusion and exclusion criteria are essential for the validity and reliability of clinical trial results. They help to ensure that the trial participants are representative of the population for whom the treatment or intervention is intended. This reduces bias and improves the generalizability of the results to the broader patient population. Furthermore, carefully defined criteria minimize the risk of enrolling participants who may not benefit from the treatment or who might be at increased risk of adverse effects.
Difference Between Inclusion and Exclusion Criteria
Inclusion criteria define the characteristics that a participant must possess to be eligible for the trial. Conversely, exclusion criteria specify characteristics that disqualify a participant from the trial. Inclusion criteria focus on characteristics that potentially benefit from the treatment, while exclusion criteria identify those who might be harmed by the treatment or whose characteristics might confound the results.
These criteria significantly impact the composition of the study population, directly influencing the study’s outcome and the applicability of the findings to real-world settings.
Examples of Inclusion Criteria
These criteria define characteristics that make a person eligible to participate in a study. They focus on specific attributes, conditions, or traits that are essential to ensure that the trial participants accurately represent the target population. For example, in a clinical trial evaluating a new drug for type 2 diabetes, inclusion criteria might specify a specific range of HbA1c levels, a particular disease stage, or a certain duration of diabetes.
- Specific disease stage (e.g., stage III lung cancer)
- Age range (e.g., 18-65 years)
- Gender (e.g., female)
- Specific type of cancer (e.g., breast cancer)
- Duration of disease (e.g., diagnosed within the last 2 years)
Examples of Exclusion Criteria
Exclusion criteria are used to identify individuals who should not be included in the study due to specific characteristics that might influence the results or put them at risk. For instance, in a study on the effectiveness of a new blood pressure medication, participants with pre-existing heart conditions might be excluded to avoid confounding the results or increasing the risk of adverse events.
- Pre-existing cardiovascular disease
- Allergy to study medication
- Pregnancy or breastfeeding
- Participation in another clinical trial
- Use of certain medications
Inclusion and Exclusion Criteria in Different Scenarios
Clinical trials in various fields often have different inclusion and exclusion criteria, tailored to the specific research question and the characteristics of the target population. The table below provides examples of these criteria in different clinical trial scenarios.
| Trial Focus | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| New anti-depressant drug | Major depressive disorder diagnosis, aged 18-65, no other significant mental health conditions | History of bipolar disorder, current use of other antidepressants, pregnancy or breastfeeding |
| New treatment for arthritis | Rheumatoid arthritis diagnosis, moderate to severe disease activity, not currently taking other arthritis treatments | Allergy to study medication, other autoimmune disorders, pregnancy or breastfeeding |
| New pain relief medication | Chronic back pain, pain level of 7 or above on a scale of 1-10, no other significant pain conditions | Previous spinal surgery, use of other pain medications with known drug interactions, severe kidney or liver problems |
Patient Recruitment Strategies
Finding the right participants is crucial for the success of any clinical trial. Recruiting a diverse and representative sample ensures the results are generalizable and applicable to a broader population. Effective recruitment strategies are essential for maintaining the integrity and validity of the research. This process involves careful planning, ethical considerations, and a variety of methods tailored to specific populations and geographical areas.
Common Recruitment Methods
Various methods are used to attract potential participants, each with its own strengths and limitations. These methods include direct outreach to specific communities, online advertising, and partnerships with healthcare providers. The chosen strategy significantly impacts the diversity of participants and must be carefully considered to ensure inclusivity and equity.
Impact on Trial Diversity
Recruitment methods play a vital role in the diversity of trial participants. For instance, relying solely on hospital-based recruitment may exclude individuals from diverse socioeconomic backgrounds or those living in rural areas. Strategies that target specific communities, such as community health centers or faith-based organizations, can increase representation from underrepresented groups. Targeted advertisements and partnerships with organizations serving marginalized communities can also improve inclusivity.
Ethical Considerations
Ethical considerations are paramount in patient recruitment. Researchers must ensure that all participants are fully informed about the trial’s purpose, procedures, risks, and benefits. Informed consent must be obtained from each individual, and their privacy must be protected. Recruitment strategies must be designed to avoid coercion or undue influence. The principles of beneficence, non-maleficence, and respect for persons must guide every aspect of the process.
Recruitment Methods
- Advertisements: Diverse advertising channels are employed, including print media, radio, television, and online platforms. The language and imagery used in advertisements must be culturally sensitive and avoid stereotypes. For example, using visuals and language that appeal to different ethnic and cultural groups can improve the recruitment rate.
- Community Outreach: This involves direct engagement with community leaders, community organizations, and local groups to raise awareness about the trial. For example, collaborating with community health workers, who are trusted members of the community, can be highly effective in recruiting participants.
- Partnerships: Collaborating with healthcare providers, research institutions, and community organizations is essential to reach a wider pool of potential participants. This can involve co-hosting events or utilizing existing networks. An example of a successful partnership is one between a university research team and a local hospital system to reach patients with a specific condition.
Geographical Variations in Recruitment
Recruitment methods differ across geographical areas due to cultural nuances and access to resources. In rural areas, community outreach and partnerships with local clinics are often more effective than online advertising. In urban settings, diverse advertising channels, including social media and community centers, are more prevalent.
Recruitment Strategies in Specific Geographical Areas
| Geographical Area | Recruitment Strategies |
|---|---|
| Rural | Community outreach, partnerships with local clinics, direct mail campaigns |
| Urban | Social media advertising, community events, partnerships with hospitals and clinics, online recruitment platforms |
| International | Collaborations with international research organizations, local community groups, translation services, culturally appropriate communication methods |
Legal and Ethical Considerations
Navigating the world of clinical trials requires a deep understanding of the legal and ethical frameworks that govern research involving human subjects. These considerations are crucial for ensuring the safety, well-being, and rights of participants while upholding the integrity of the scientific process. Ethical conduct is paramount in trials, ensuring equitable access to potential treatments and fostering public trust in medical research.A robust framework of legal and ethical principles is vital for safeguarding participants’ rights and ensuring the ethical conduct of clinical trials.
Eligibility for clinical trials varies widely, depending on the specific trial and the condition being studied. Before you even consider participating, you need to understand the criteria. To get the most out of a meeting with the clinical trials research coordinator or doctor, proper preparation is key. For example, having a list of questions ready will help ensure you understand the requirements and any potential risks.
Reviewing how should i prepare for a meeting with the clinical trials research coordinator or doctor will give you an idea of what to expect. Ultimately, understanding your own health status and potential suitability for a particular trial is crucial for making an informed decision.
This framework protects vulnerable populations, promotes informed decision-making, and maintains the integrity of research findings. Clear guidelines and procedures are essential to uphold the trust and confidence of participants and the wider community in the integrity of medical research.
Legal Regulations Governing Clinical Trial Participation
Various legal regulations govern clinical trial participation, ensuring the protection of participants’ rights and the ethical conduct of research. These regulations are often established at a national or international level. Compliance with these regulations is critical to maintain public trust and uphold the highest standards of research integrity. These regulations often address informed consent, data privacy, and the role of institutional review boards.
Informed Consent
Informed consent is a cornerstone of ethical clinical trial conduct. It’s a process that ensures participants fully understand the risks, benefits, and procedures involved in the trial. This process is vital in allowing participants to make autonomous decisions about their participation. Obtaining informed consent involves providing clear, comprehensive information about the study, enabling participants to comprehend the potential consequences of their involvement.
Role of Institutional Review Boards (IRBs)
Institutional Review Boards (IRBs) play a critical role in ensuring the ethical conduct of clinical trials. These independent committees review research protocols to assess the risks and benefits to participants and ensure compliance with ethical guidelines. They act as an oversight body, evaluating the ethical implications of research designs and procedures, protecting the rights and safety of participants.
Clinical trials often have specific eligibility criteria, so not everyone can participate. For instance, a recent study, highlighting the best approaches for weight loss in individuals with depression , likely had strict inclusion and exclusion criteria. This means researchers carefully select participants to ensure the study’s integrity and the results’ reliability. Ultimately, the criteria depend on the specific trial, but it’s important to be aware of the requirements before considering joining.
Data Privacy and Confidentiality Protocols
Data privacy and confidentiality are paramount in clinical trials. Protocols must be in place to safeguard sensitive participant data. These protocols are critical to maintain participant trust and confidentiality. Strict adherence to these protocols is essential to avoid breaches and maintain the integrity of the research.
Legal and Ethical Frameworks in the USA
| Legal Framework | Description |
|---|---|
| The Common Rule (45 CFR 46) | This federal regulation Artikels the ethical principles for research involving human subjects, including informed consent, risk-benefit assessment, and the role of IRBs. |
| Food and Drug Administration (FDA) regulations | Specific regulations related to the conduct of clinical trials for drugs and medical devices. These regulations ensure that trials are conducted safely and effectively. |
| State laws | Some states may have additional regulations or requirements for clinical trials conducted within their borders. |
| HIPAA (Health Insurance Portability and Accountability Act) | This legislation protects the privacy and security of individually identifiable health information. |
Communicating Trial Information to Potential Participants
Reaching out to potential participants for clinical trials requires careful consideration of how information is conveyed. Clear, concise, and culturally sensitive communication is paramount to ensuring informed consent and building trust. This process needs to be more than just a transaction; it should be a partnership where participants feel empowered and understood.Effective communication strategies are essential for successful recruitment, enabling potential participants to make informed decisions about their health and well-being.
This involves understanding the nuances of various cultural backgrounds, ensuring accessibility for individuals with diverse needs, and using language that is easy to grasp.
Clear and Concise Language
Communicating complex medical information requires simplifying technical jargon and presenting details in plain language. This approach ensures that potential participants understand the purpose, procedures, and risks associated with the trial. Using everyday language, avoiding medical terminology unless absolutely necessary, and providing visual aids, such as diagrams or flowcharts, can significantly enhance comprehension. Examples include avoiding phrases like “randomized controlled trial” and instead using simpler terms like “a trial where participants are put into different groups.” Detailed explanations of the trial’s objectives, procedures, and potential outcomes should be presented in a straightforward and easily understandable format.
Tailoring Communication to Diverse Populations
Different cultural backgrounds and communication styles necessitate tailored approaches. For instance, individuals from diverse linguistic backgrounds require materials translated into their native languages. Consider using culturally relevant imagery and examples in educational materials to enhance understanding and connection. Community engagement plays a critical role in facilitating outreach and ensuring that the target population receives information in a way that is accessible and meaningful to them.
Clinical trials are fascinating, but who exactly gets to participate? Well, eligibility criteria vary widely, depending on the specific trial. For example, researchers are looking into a potential link between certain herpes infections and cognitive decline, like dementia. Recent studies have shown some compelling evidence of this link, as explored further in this article about evidence of link between herpes and dementia.
Ultimately, it boils down to meeting specific health requirements and being a good fit for the study’s goals, ensuring the data collected is reliable and useful. So, if you’re curious about participating, check out the criteria carefully!
For example, collaborating with community leaders or faith-based organizations to distribute information can significantly increase the reach and impact of communication efforts.
Effective Communication Strategies for Diverse Populations
Understanding and respecting the diverse needs of potential participants is crucial. This includes addressing the unique communication styles and preferences of different cultural groups. For example, some cultures may prefer face-to-face interactions, while others might favor written materials or online resources.
- Language Accessibility: Provide translated materials in multiple languages. Utilize multilingual staff or interpreters to facilitate communication with individuals who do not speak the dominant language.
- Cultural Sensitivity: Ensure that materials and interactions are sensitive to cultural norms and beliefs. For instance, avoid potentially offensive or inappropriate imagery and language.
- Community Engagement: Partner with community organizations and leaders to build trust and enhance access to information. This might involve holding community meetings or workshops.
- Accessibility Considerations: Provide information in accessible formats for individuals with visual, auditory, or cognitive impairments. This may involve providing large-print materials, audio recordings, or braille versions of the trial information.
Example of a Language-Neutral Pamphlet
This example pamphlet focuses on clarity and simplicity, using visuals to support the text. It Artikels the key elements of a hypothetical trial for treating a common ailment. The language is intentionally neutral, avoiding overly technical terms.
| Section | Content |
|---|---|
| Trial Overview | This trial studies a new treatment for common headaches. It aims to find out if the new treatment is better than the current standard treatment. |
| Who Can Participate? | Healthy adults with a history of frequent headaches can participate. |
| What to Expect |
|
| Potential Risks | All treatments have potential side effects. Common side effects may include mild nausea or dizziness. Serious side effects are rare. |
| How to Enroll | Contact the trial coordinator for more details and to express your interest. |
Participant Support and Resources

Navigating a clinical trial can be a complex process, and providing comprehensive support is crucial for participant well-being and successful trial completion. This section Artikels the support systems in place to ensure participants feel supported throughout their journey. Understanding the resources available can ease anxieties and facilitate a positive experience.Trial participation often involves a commitment, and a supportive environment is paramount.
This support extends beyond the immediate trial procedures and encompasses the entire experience. Resources are tailored to meet individual needs, recognizing that each participant’s experience is unique.
Support Systems for Trial Participants
The trial team provides a range of support systems to ensure participants feel comfortable and well-informed. This includes regular check-ins, access to educational materials, and a dedicated point of contact for addressing questions or concerns.
- Regular Check-ins: Scheduled communication with participants helps track their progress, address any emerging issues, and ensure ongoing engagement with the trial. This proactive approach helps identify potential problems early and facilitates timely intervention. For instance, participants might receive regular phone calls or email updates to discuss their experience and provide ongoing support.
- Educational Resources: Access to comprehensive information about the trial, its procedures, and expected outcomes is vital. This includes brochures, pamphlets, and online resources, offering clear and concise explanations. These materials address questions participants might have and help ensure a good understanding of the process.
- Dedicated Contact Person: A designated individual serves as a point of contact for participants, providing a direct line of communication for questions, concerns, and feedback. This individual is readily available to address queries promptly and efficiently. This direct support network fosters trust and confidence in the trial process.
Addressing Participant Concerns and Questions
A robust system for addressing participant concerns and questions is integral to the success of a clinical trial. The system ensures prompt responses and provides support in a respectful and understanding manner.
- Prompt Response Mechanisms: The trial team employs various methods to ensure rapid responses to participant inquiries. This could involve email, phone calls, or online forums, all designed to provide timely and efficient communication.
- Clear Communication Channels: Participants are provided with detailed instructions on how to reach the support team, ensuring clarity and ease of access. This information is clearly Artikeld in the participant information sheets, providing participants with multiple options for communication.
- Confidentiality and Privacy: The utmost importance is placed on maintaining the confidentiality and privacy of all participant information. Strict adherence to data protection regulations is essential.
Support for Participants in Remote Areas
Participants residing in remote locations require specific considerations to ensure equitable access to trial support.
- Remote Access to Resources: Participants in remote areas are provided with access to online resources, educational materials, and communication channels. This includes ensuring access to technology for online support and virtual meetings.
- Telemedicine Options: Telemedicine consultations and remote monitoring are incorporated where feasible to provide access to medical support and ongoing assessment without geographical limitations.
- Transportation Assistance: If necessary, participants in remote areas may be offered transportation assistance to attend appointments and receive care.
Process for Addressing Concerns in a Timely and Effective Manner
A well-defined process is crucial for addressing participants’ concerns in a timely and effective manner.
- Clear Escalation Procedures: A clear escalation path is established to ensure that concerns are addressed at appropriate levels within the organization. This helps facilitate a timely resolution to participant issues.
- Feedback Mechanisms: Regular feedback mechanisms are in place to gather input from participants on the effectiveness of the support systems. This ongoing feedback loop allows for continuous improvement and adaptation of support services.
- Evaluation of Resolution Effectiveness: The trial team assesses the effectiveness of their response to participant concerns to identify areas for improvement. This ongoing evaluation ensures the system adapts to meet the evolving needs of participants.
Diverse Populations and Inclusion: Who Can Participate In A Clinical Trial
Clinical trials are crucial for advancing medical knowledge and improving patient care. However, their effectiveness hinges on the representation of diverse populations. Without inclusive participation, the results may not be generalizable to all patient groups, potentially leading to treatments that are less effective or even harmful for certain demographics. This section will explore the significance of diverse populations in trials, the barriers to participation, and strategies to overcome these obstacles.
Importance of Diversity in Clinical Trials
Clinical trials must reflect the diversity of the patient population they aim to treat. This ensures that the efficacy and safety of a treatment are assessed in a broader context, encompassing various ethnicities, socioeconomic backgrounds, and health conditions. Studies have shown that treatments that appear effective in a homogenous group may not yield the same results in a more diverse population.
Understanding how treatments affect different groups allows researchers to develop more targeted and personalized medicine.
Barriers to Participation for Specific Demographic Groups
Several factors hinder participation in clinical trials for specific demographic groups. Language barriers, cultural beliefs, socioeconomic constraints, and lack of trust in the healthcare system can create significant hurdles. For instance, patients from lower socioeconomic backgrounds may face difficulties in affording transportation, childcare, or lost wages during trial participation. Furthermore, cultural sensitivity is paramount; patients may be hesitant to participate if the trial protocols or procedures do not align with their cultural values or beliefs.
Limited access to healthcare, including information about trials, is another significant factor.
Overcoming Barriers to Ensure Diverse Representation
Strategies for overcoming barriers to participation require a multifaceted approach. First, researchers must actively recruit participants from underrepresented groups. This can involve partnerships with community organizations, healthcare providers, and faith-based institutions. Clear and accessible communication in multiple languages is essential. Simplifying trial procedures and offering financial incentives, such as transportation allowances and compensation for time lost, can increase participation.
Building trust with communities is critical; researchers should engage in open dialogue, address concerns, and demonstrate respect for cultural nuances.
Strategies to Improve Trial Participation Among Underrepresented Groups
Effective strategies include offering culturally sensitive information materials, providing multilingual support, and collaborating with community leaders. Offering incentives like transportation assistance, childcare, and compensation for lost wages can make participation more feasible for individuals from disadvantaged backgrounds. Utilizing community health workers who are fluent in the local language and understand cultural nuances can also significantly improve participation rates.
Trials should also be designed to accommodate diverse schedules and needs.
Designing Inclusive Trials for Various Cultural and Socioeconomic Backgrounds
Inclusive trial design necessitates careful consideration of various factors. Trial materials should be translated into multiple languages and presented in culturally sensitive formats. The trial environment should be welcoming and respectful of different cultural practices. Researchers must be sensitive to the potential impact of socioeconomic factors on participation. For example, offering flexible scheduling and providing transportation assistance can make participation more accessible to those with limited resources.
Furthermore, ensuring that the research team includes members from diverse backgrounds can enhance cultural understanding and sensitivity.
Concluding Remarks
In conclusion, participation in a clinical trial is a significant decision. The process involves a range of factors, from eligibility criteria to ethical considerations and participant support. Understanding these elements allows potential participants to make informed choices and ensures the trial’s success. Ultimately, careful consideration of the specific criteria and support systems available is essential for a positive and productive experience for everyone involved.
Diversity and inclusion are key aspects in clinical trials, and ongoing efforts to overcome barriers to participation are crucial for ensuring reliable and representative research outcomes.