Promising treatments clinical trials are revolutionizing medicine, offering hope for countless patients. These meticulously designed studies meticulously evaluate new therapies, from early-stage research to late-phase testing. Understanding the process, from identifying potential treatments to assessing efficacy and safety, is crucial for informed decision-making. This exploration delves into the intricacies of clinical trials, examining the methods, challenges, and future implications.
Clinical trials encompass various phases, each with a distinct purpose. Early phases focus on safety and initial effectiveness, while later phases rigorously evaluate long-term outcomes and efficacy. This systematic approach allows researchers to identify promising treatments with a high degree of confidence. Different trial designs, like randomized controlled trials and observational studies, provide unique insights into the efficacy of interventions.
Introduction to Promising Treatments in Clinical Trials
Clinical trials are the cornerstone of medical advancement, meticulously designed experiments that evaluate the safety and effectiveness of new treatments, diagnostic tools, and preventive measures. They provide crucial data for determining whether a treatment is truly beneficial and safe for patients, moving beyond the confines of laboratory research into real-world application. Without rigorous clinical trials, the transition from promising research to established medical practice would be significantly hampered.Clinical trials meticulously assess the impact of new interventions, meticulously examining their effectiveness and potential risks.
This systematic approach ensures that treatments are evaluated under controlled conditions, reducing the influence of extraneous factors and increasing the reliability of the results.
Phases of Clinical Trials
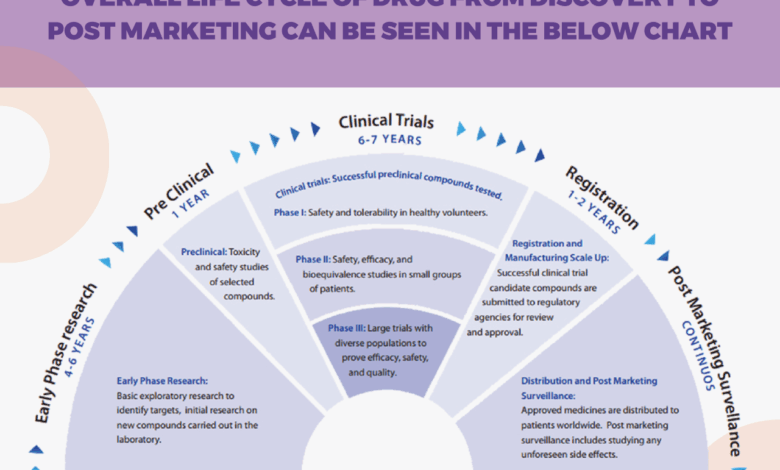
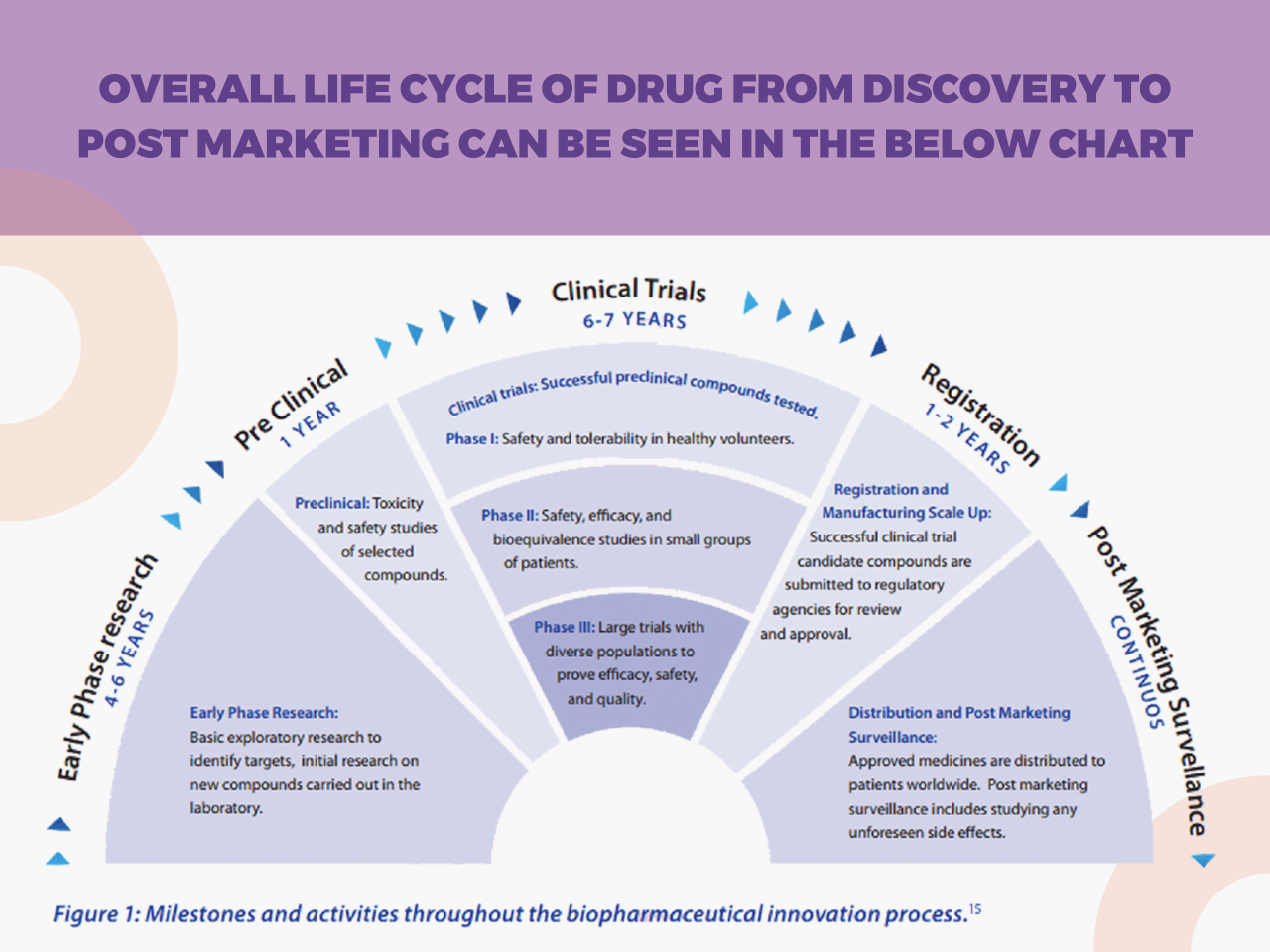
Clinical trials are conducted in phases, each with a distinct purpose. Understanding these phases is crucial for comprehending the progression of a treatment from initial investigation to potential widespread use.Phase 1 trials primarily focus on establishing the safety of a new treatment in a small group of healthy volunteers or patients with a specific disease. The primary goal is to identify potential side effects and determine the appropriate dosage.Phase 2 trials expand the scope, enrolling a larger group of patients with the target disease.
The focus shifts to evaluating the treatment’s efficacy and further refining the dosage.Phase 3 trials involve a larger cohort of patients and are designed to confirm the treatment’s efficacy and compare it to existing standard treatments. Statistical significance is crucial in this phase.Phase 4 trials monitor the long-term effects of a treatment after it has been approved and is in widespread use.
Criteria for Identifying Promising Treatments
Several factors contribute to the identification of promising treatments for clinical trials. These treatments often exhibit efficacy in preclinical studies, demonstrating promising results in laboratory settings or animal models. Furthermore, existing evidence from previous studies, either observational or experimental, can indicate a potential benefit. Finally, the potential for improving patient outcomes and addressing unmet medical needs plays a crucial role.
Types of Clinical Trial Designs
Different clinical trial designs employ varying methodologies to assess the effectiveness of interventions. Understanding these differences is essential for evaluating the robustness of the study findings.
Clinical trials are exploring promising new treatments for various conditions. One area of interest is black seed oil for eczema, with some studies showing potential benefits. Black seed oil for eczema might offer a natural approach, but further research is needed to fully understand its effectiveness and safety. These ongoing trials are crucial to advancing our knowledge and ultimately leading to more effective and accessible treatments.
| Trial Design | Description | Strengths | Limitations |
|---|---|---|---|
| Randomized Controlled Trials (RCTs) | Participants are randomly assigned to either an experimental group receiving the new treatment or a control group receiving a standard treatment or placebo. | High internal validity, minimizing bias, and allowing for causal inferences. | Can be expensive and time-consuming, may not always reflect real-world situations. |
| Observational Studies | Researchers observe and collect data on participants without manipulating any variables. | Can be less expensive and faster than RCTs, often suitable for exploring relationships between factors in real-world settings. | Lower internal validity, potentially influenced by confounding variables, and cannot establish causal relationships. |
Identifying Emerging Therapies
Unveiling novel treatments is a dynamic process, often driven by innovative research and rigorous testing. Clinical trials play a crucial role in validating promising therapies, moving them from the lab to real-world applications. Understanding the factors behind the identification of these therapies, the vital role of preclinical research, and the emerging therapies currently in development is essential for informed discussions about the future of healthcare.Identifying promising treatments involves a multi-faceted approach.
Promising treatments are emerging from clinical trials, offering hope for managing various health issues. However, the booming secondary drug industry, fueled by the opioid epidemic, likewise highlights the complex interplay of factors affecting treatment accessibility and affordability. Ultimately, these advancements in promising treatments clinical trials are crucial for a healthier future.
A combination of scientific intuition, meticulous data analysis, and the integration of diverse research findings are instrumental in pinpointing potential therapeutic targets. A deep understanding of disease mechanisms, coupled with the identification of specific biological pathways or molecular targets, forms the bedrock of this process.
Factors Contributing to Identification
Several factors contribute to the identification of promising treatments. These include a comprehensive understanding of the disease’s pathophysiology, insights from basic science research, and the development of novel technologies that allow for more precise identification of disease targets. Strong statistical significance in preclinical studies, coupled with the safety profile observed in these earlier stages, further enhance the potential of a treatment.
The potential for broad applicability and cost-effectiveness are also key considerations.
Role of Preclinical Research
Preclinical research is pivotal in identifying potential therapeutic targets. This stage involves testing potential treatments in laboratory settings using cells, tissues, and animal models. The findings from these studies help determine the safety and efficacy of a treatment before it’s tested in humans. This reduces the risk of adverse events in clinical trials and increases the chances of success.
Early validation of a treatment’s mechanism of action through preclinical studies significantly strengthens the case for further investigation.
Examples of Emerging Therapies
Several emerging therapies show promise in early-stage trials. For instance, targeted therapies, which specifically target cancer cells without harming healthy cells, are demonstrating encouraging results. Immunotherapies, which harness the body’s immune system to fight disease, are also showing great potential. Gene therapies, which aim to correct genetic defects responsible for diseases, are another promising area of development.
The identification of novel biomarkers that can predict treatment response and identify patients most likely to benefit from a specific treatment is another key factor in the development of emerging therapies.
Characteristics of Promising Treatments
| Disease Area | Mechanism of Action | Stage of Development |
|---|---|---|
| Cancer (Lung Cancer) | Targeted inhibition of specific growth factors | Phase II |
| Neurodegenerative Disorders (Alzheimer’s Disease) | Modulation of amyloid-beta aggregation | Phase I |
| Infectious Diseases (Viral Hepatitis) | Novel antiviral agents | Phase I/II |
| Autoimmune Diseases (Rheumatoid Arthritis) | Immunomodulation | Phase II |
This table provides a snapshot of promising treatments currently under investigation, highlighting their respective disease areas, mechanisms of action, and the stage of clinical development. The diversity of conditions and approaches underscores the breadth of research efforts focused on identifying and developing new treatments. Further research and clinical trials are essential to confirm efficacy and safety.
Evaluation of Treatment Efficacy and Safety
Unveiling the promise of new treatments hinges on meticulous evaluation of their efficacy and safety. Clinical trials meticulously document how well these treatments work and whether they cause harmful side effects. This process, while complex, is crucial for determining the true value and potential risks associated with any new therapy.Evaluating treatment efficacy and safety in clinical trials involves a structured approach, employing rigorous methodologies and standardized outcome measures.
These measures allow researchers to objectively assess the impact of a treatment on patients and compare it to existing treatments or a placebo.
Methods for Assessing Efficacy and Safety
Clinical trials utilize various methods to evaluate treatments, from analyzing patient responses to monitoring adverse events. Researchers meticulously collect data from multiple sources, including patient reports, medical examinations, and laboratory tests. Data analysis is performed using statistical techniques to determine if observed effects are statistically significant and not due to chance.
Outcome Measures in Clinical Trials
A wide range of outcome measures are employed in clinical trials to assess treatment efficacy and safety. These measures can be categorized into primary and secondary endpoints, each playing a distinct role in evaluating the treatment’s impact. Primary endpoints directly measure the treatment’s effectiveness, while secondary endpoints explore other potential benefits or risks. This comprehensive approach provides a more complete picture of the treatment’s profile.
Types of Endpoints Used to Measure Efficacy and Safety
- Primary Endpoints: These are the most crucial measurements directly reflecting the treatment’s effectiveness. For example, in a trial evaluating a new drug for a specific disease, a primary endpoint might be the reduction in disease symptoms, measured using a validated scale. The magnitude of the observed effect is statistically analyzed to determine if the treatment is superior to a control group or placebo.
- Secondary Endpoints: These endpoints provide further insights into the treatment’s impact. Examples include changes in quality of life, duration of symptom relief, and the frequency of adverse events. Secondary endpoints often provide a broader context for the treatment’s overall effect, supplementing the findings of primary endpoints.
- Safety Endpoints: These measurements focus on the potential adverse effects of the treatment. This includes monitoring for side effects, such as allergic reactions, organ damage, or changes in blood parameters. These data help determine the treatment’s safety profile and inform dosage adjustments or potential contraindications. For example, a trial evaluating a new chemotherapy drug would closely monitor for hematological toxicity (blood abnormalities).
This careful monitoring is crucial for preventing serious adverse events.
Data Analysis and Evaluation of Treatment Effectiveness and Risks
Data from clinical trials are meticulously analyzed to evaluate the effectiveness and risks of a treatment. Statistical analysis methods are applied to compare the treatment group to control groups, determining if the observed effects are statistically significant. Statistical significance, however, does not automatically translate to clinical significance. Clinical significance refers to the practical importance of the observed effect. For example, a statistically significant but minor reduction in blood pressure may not be clinically relevant.
The analysis process also involves considering factors such as patient demographics, baseline disease severity, and adherence to the treatment protocol. These considerations help researchers identify potential confounding variables and ensure that observed effects are attributable to the treatment itself. Furthermore, data is scrutinized for any trends or patterns suggestive of adverse effects. This data-driven approach is essential for evaluating the treatment’s overall impact, allowing researchers to assess its potential benefits and risks.
| Endpoint Category | Example Endpoints | Measurement Method |
|---|---|---|
| Efficacy | Tumor shrinkage, reduction in pain scores, improvement in blood pressure | Clinical scales, imaging studies, laboratory tests |
| Safety | Incidence of adverse events, changes in blood parameters, organ function tests | Patient questionnaires, medical examinations, laboratory tests |
Challenges and Considerations in Clinical Trials: Promising Treatments Clinical Trials

Clinical trials, while crucial for advancing medical knowledge and developing life-saving treatments, are not without their complexities. Navigating ethical considerations, participant recruitment, financial constraints, and potential biases is essential for ensuring the validity and reliability of the results. These factors can significantly impact the success of a trial and the ultimate impact on patients.
Ethical Considerations in Clinical Trials, Promising treatments clinical trials
Ethical considerations are paramount in clinical trials. Researchers must prioritize the well-being and rights of participants throughout the entire process. Informed consent, a cornerstone of ethical research, ensures participants understand the study’s purpose, procedures, potential risks and benefits, and their right to withdraw at any time. Confidentiality of personal data is also a critical ethical concern, requiring rigorous measures to protect sensitive information.
Ensuring equitable access to experimental treatments and avoiding exploitation of vulnerable populations are equally important ethical principles. Trial designs must adhere to strict ethical guidelines and oversight by institutional review boards (IRBs) to protect participants from harm.
Challenges in Recruiting Participants for Clinical Trials
Recruiting sufficient and diverse participants for clinical trials can be a significant hurdle. Several factors contribute to this challenge. Lack of awareness about clinical trials within the target population, geographical limitations, and logistical barriers can all impede recruitment. Furthermore, some individuals may be hesitant to participate due to concerns about the potential risks involved, the inconvenience of trial procedures, or mistrust of research institutions.
Addressing these barriers requires targeted recruitment strategies, clear communication, and building trust with potential participants. For example, online platforms and community outreach programs can enhance awareness and facilitate participation.
Financial Constraints Impacting Treatment Development
The development of promising treatments is often hampered by substantial financial constraints. Clinical trials require significant investment in research personnel, infrastructure, equipment, and data analysis. Limited funding can lead to delays in trial initiation, reduced sample sizes, and inadequate resources for data management. This can compromise the reliability of the results and potentially delay the availability of effective treatments for patients.
Further complicating matters, the cost of bringing a new drug to market can be enormous, often exceeding hundreds of millions of dollars. This economic burden can dissuade pharmaceutical companies from pursuing promising but costly research avenues.
Biases Affecting Clinical Trial Results
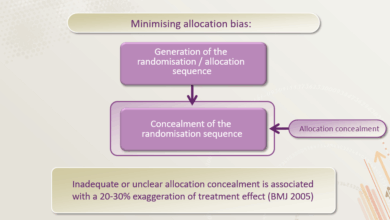
Biases can inadvertently influence clinical trial results, leading to inaccurate conclusions. Selection bias occurs when participants in a trial are not representative of the target population. This can arise from various factors, such as exclusion criteria or unequal access to participation. Another critical issue is performance bias, where knowledge of treatment assignment influences the outcome. For instance, if clinicians administering treatment are aware of which participants are receiving the experimental therapy, their behavior could inadvertently affect the trial results.
Furthermore, detection bias arises when the outcome assessment differs based on knowledge of the treatment. This can lead to inaccurate or exaggerated results. Minimizing these biases requires meticulous study design, careful participant selection, and rigorous data analysis procedures.
Public Perception and Understanding of Clinical Trials
Public trust in medical research, particularly clinical trials, is crucial for their success. A well-informed and engaged public is more likely to participate in trials, contributing valuable data and potentially benefiting from new treatments. However, misconceptions and lack of understanding can hinder recruitment and ultimately impact the development of life-saving therapies. This section explores the factors influencing public trust, communication strategies used to educate the public, and effective methods for disseminating information.Public perception of clinical trials is shaped by a complex interplay of factors.
Exciting clinical trials are uncovering promising new treatments for various health conditions. While some promising treatments are still in the early stages, researchers are exploring innovative therapies. For example, some studies are looking at the potential of coconut oil for cellulite, like this one coconut oil for cellulite. These investigations are essential to further develop and refine effective treatments for a wide range of conditions.
These include prior experiences with medical interventions, media portrayals, and personal biases. Positive experiences with healthcare providers and institutions often foster trust, while negative or sensationalized media coverage can erode confidence.
Factors Influencing Public Trust and Engagement
Public trust in clinical trials is influenced by several key factors. Transparency in research methodologies, clear communication about potential risks and benefits, and a sense of patient autonomy are vital for building confidence. Furthermore, the perceived value of the research and the benefits to future patients are critical considerations. The public’s perception of the institutions conducting the trials also plays a significant role.
Credibility and demonstrated commitment to ethical practices are essential for establishing trust. For instance, clinical trials conducted by reputable hospitals or universities often garner greater public trust than those conducted by less established entities.
Communication Strategies for Educating the Public
Effective communication strategies are essential for educating the public about clinical trials. These strategies need to be tailored to the specific audience, considering their existing knowledge, concerns, and cultural backgrounds. Clear and concise explanations of the trial process, including the purpose, procedures, and potential outcomes, are vital. Active engagement through interactive platforms, community events, and educational materials can foster a deeper understanding.
For example, online forums and social media groups dedicated to specific conditions can be used to disseminate information about relevant trials and encourage participation.
Methods for Disseminating Information
Various methods can be used to disseminate information about clinical trials to the public. Utilizing trusted channels, such as patient advocacy groups, healthcare providers, and reputable news outlets, is crucial. Educational materials, including brochures, pamphlets, and online resources, can provide accessible and detailed information. Public service announcements (PSAs) on television, radio, and online platforms can reach a wider audience, increasing awareness and dispelling myths.
Furthermore, partnering with influencers or celebrities who have personal experience with the condition being researched can amplify the message and increase public interest.
Comparing and Contrasting Public Engagement Methods
Different methods of public engagement have varying strengths and weaknesses. For example, interactive webinars and online Q&A sessions can foster direct interaction and address concerns in real-time. However, these methods might not reach as broad a spectrum of the public compared to social media campaigns or print materials. In contrast, while print materials offer detailed information, they may not offer the same level of immediacy or interactive feedback as online platforms.
Understanding these nuances is key to developing a comprehensive and effective communication strategy. Utilizing a multi-faceted approach, combining multiple channels, is often the most effective way to reach the widest possible audience and promote public engagement in clinical trials.
Future Directions and Implications
The burgeoning field of promising treatments, as evidenced by clinical trials, holds immense potential to revolutionize healthcare. Understanding the potential impact on healthcare systems, the long-term consequences of these therapies, and the future directions of clinical trials themselves is crucial for responsible implementation and societal benefit. This exploration delves into the implications of these advancements.The potential impact of these treatments extends beyond individual patient care to encompass significant societal implications.
Successful therapies can reduce healthcare costs associated with long-term management of diseases by improving outcomes and potentially preventing disease progression. However, widespread adoption of novel treatments necessitates careful consideration of their potential long-term effects.
Potential Impact on Healthcare Systems
The widespread adoption of promising treatments will undoubtedly place a strain on healthcare systems, particularly regarding resource allocation. Hospitals and clinics may need to adapt their infrastructure, training programs, and supply chains to accommodate new therapies. The cost-effectiveness of these treatments will be a critical factor in their integration into standard care protocols. Furthermore, the potential for reduced healthcare costs associated with improved outcomes and reduced hospital stays needs careful analysis.
Potential Long-Term Consequences of New Therapies
Long-term effects of new therapies are not always immediately apparent. Unforeseen side effects or interactions with other medications can emerge over time. This underscores the importance of long-term follow-up studies and comprehensive safety monitoring. Real-world examples of new drugs causing unexpected long-term complications, such as certain cardiovascular drugs, highlight the necessity of ongoing research and monitoring.
Future Research Directions for Advancing Clinical Trial Methodologies
The evolution of clinical trials must keep pace with the increasing complexity of new therapies. Improving recruitment strategies for diverse populations, utilizing advanced statistical methods to analyze large datasets, and implementing real-world evidence frameworks are key areas for future research. This will enhance the accuracy and reliability of clinical trial results and ensure the treatments reach those who need them most.
Potential Future Applications of Promising Treatments
| Medical Field | Potential Applications |
|---|---|
| Oncology | Targeted therapies for specific cancer types, immunotherapies, personalized medicine approaches, and innovative combination therapies. |
| Neurology | Developing treatments for neurodegenerative diseases like Alzheimer’s and Parkinson’s, improving stroke recovery, and addressing neurological disorders with innovative cellular therapies. |
| Cardiology | New approaches to heart failure management, advanced cardiac resynchronization therapy, and the development of novel therapies for preventing and treating cardiovascular diseases. |
| Infectious Diseases | Development of more effective and targeted antivirals and antibacterial agents, potential for prophylactic treatments, and improved strategies to combat emerging infectious threats. |
| Immunology | Novel immunomodulatory therapies for autoimmune diseases, treatments for primary immunodeficiencies, and advanced cancer immunotherapies. |
Final Review
In conclusion, promising treatments clinical trials represent a vital cornerstone of medical advancement. The careful evaluation of new therapies, coupled with rigorous ethical considerations and public engagement, is essential for maximizing the benefits of medical innovation. Future research should continue to refine trial methodologies and ensure equitable access to these transformative treatments, ultimately impacting healthcare systems and improving patient lives.