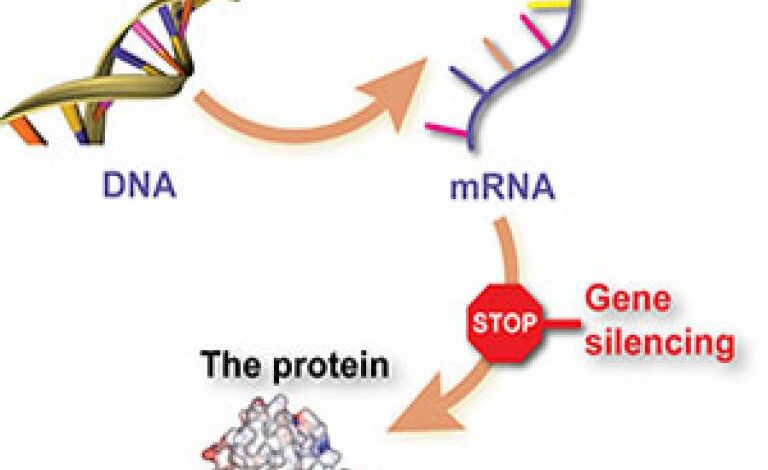
New drug for huntingtons disease – New drug for Huntington’s disease is a beacon of hope for those battling this devastating neurological disorder. This in-depth exploration delves into the current state of research, examining promising new targets, clinical trial methodologies, potential benefits and risks, and future research priorities. We’ll navigate the complex landscape of Huntington’s disease, considering both the excitement surrounding potential breakthroughs and the hurdles that remain.
The current understanding of Huntington’s disease, encompassing its genetic basis, symptoms, and progression, will be discussed. We’ll explore the major challenges and limitations in developing effective treatments and the existing therapeutic strategies, while comparing efficacy and safety profiles of different drug candidates in clinical trials. This comprehensive analysis will also include a breakdown of the stages of drug development, from preclinical testing to regulatory approvals.
Furthermore, the discussion will highlight emerging drug targets, clinical trial design, potential benefits and risks, and future research directions.
Current Status of Huntington’s Disease Research
Huntington’s disease (HD) is a devastating neurodegenerative disorder characterized by progressive motor, cognitive, and psychiatric symptoms. Understanding its underlying mechanisms and developing effective treatments remain significant challenges in medical research. This exploration delves into the current understanding of HD, highlighting the hurdles in treatment development and the existing therapeutic strategies.HD is a genetic disorder caused by a mutation in the HTT gene, leading to the production of an abnormal huntingtin protein.
This abnormal protein aggregates in neurons, disrupting cellular function and eventually causing neuronal death. The symptoms of HD typically emerge in adulthood, ranging from subtle personality changes to severe chorea (involuntary movements), cognitive decline, and psychiatric disturbances. The disease progression varies greatly among individuals, but it invariably leads to significant disability and ultimately death.
Current Understanding of Huntington’s Disease
Huntington’s disease is characterized by a complex interplay of genetic, cellular, and molecular events. The mutation in the HTT gene results in an expanded CAG trinucleotide repeat sequence. The length of this repeat correlates with the age of onset and severity of the disease. This genetic basis allows for early diagnosis through genetic testing. The abnormal huntingtin protein disrupts various cellular processes, including protein transport, synaptic function, and neuronal survival.
The progressive neuronal loss, particularly in the striatum and cortex of the brain, leads to the characteristic symptoms of the disease.
Challenges in Developing Treatments
Several obstacles hinder the development of effective treatments for HD. The progressive nature of the disease makes it difficult to determine the optimal time for intervention. Early diagnosis, while possible, is not always accompanied by clear early symptoms. Another significant challenge lies in the complexity of the disease’s pathophysiology. The intricate interplay of genetic, cellular, and molecular factors contributes to the multifaceted nature of HD, making it difficult to target specific mechanisms effectively.
Existing Therapeutic Strategies and Limitations
Currently, there are no cures for Huntington’s disease. Existing therapies primarily focus on managing symptoms. Pharmacological approaches aim to alleviate motor symptoms (such as chorea) and address psychiatric issues. For instance, medications like tetrabenazine are used to reduce involuntary movements. However, these treatments often have limited efficacy and can cause side effects.
Other strategies, including neuroprotective agents, aim to slow disease progression by targeting the underlying mechanisms of neuronal damage. However, clinical trials of these agents have often yielded disappointing results.
Exciting news on the new drug front for Huntington’s disease! Researchers are making strides, but managing the underlying causes of this devastating condition still presents a huge challenge. Perhaps, like understanding our own cravings, taking a self-assessment quiz like the quiz addicted to sugar could help us better grasp the complexities of our own bodies and behaviors.
Ultimately, though, continued research and development are crucial in the fight against Huntington’s disease.
Efficacy and Safety Profile Comparison of Drug Candidates
| Drug Candidate | Efficacy (reduction in symptoms) | Safety Profile (side effects) | Phase of Clinical Trials ||—|—|—|—|| Example Drug 1 | Moderate | Mild | Phase II || Example Drug 2 | Low | Severe | Phase I || Example Drug 3 | High | Moderate | Phase III |Note: This table provides illustrative examples and should not be interpreted as a definitive assessment of all drug candidates.
Data on efficacy and safety are subject to change based on ongoing clinical trials.
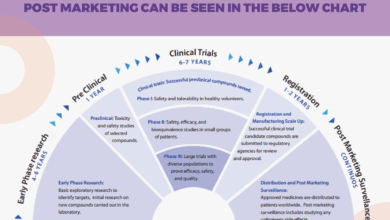
Stages of Drug Development for Huntington’s Disease
| Stage | Description ||—|—|| Preclinical Testing | Involves laboratory experiments, animal models, and cell cultures to assess the potential efficacy and safety of a drug candidate. || Clinical Trials (Phase I) | Involves a small group of healthy volunteers to evaluate the safety and tolerability of the drug. || Clinical Trials (Phase II) | Involves a larger group of patients to assess the drug’s effectiveness and identify optimal dosages.
|| Clinical Trials (Phase III) | Involves a large group of patients to confirm the drug’s efficacy, safety, and optimal dosing. || Regulatory Approvals | Involves submission of data to regulatory agencies for review and approval. |
Emerging Drug Targets and Mechanisms
Huntington’s disease (HD) is a devastating neurodegenerative disorder characterized by progressive motor, cognitive, and psychiatric symptoms. Current therapeutic strategies primarily focus on managing symptoms, rather than halting or reversing disease progression. This necessitates a continued exploration of novel drug targets and mechanisms to effectively combat the underlying pathological processes. New avenues of research are crucial to improving the lives of those affected by HD.Beyond the current focus on inhibiting mutant huntingtin protein aggregation, researchers are actively investigating alternative pathways and targets involved in HD pathogenesis.
These include mechanisms related to neuroinflammation, oxidative stress, and altered cellular metabolism, promising novel therapeutic avenues. The goal is to develop treatments that address the multifaceted nature of HD, rather than just one aspect.
Potential New Drug Targets
Several potential new drug targets are emerging beyond the traditional focus on mutant huntingtin protein. These include proteins involved in neuroinflammation, such as cytokines and inflammatory mediators. Another promising area is targeting oxidative stress pathways, as the accumulation of reactive oxygen species (ROS) can contribute to neuronal damage. Additionally, cellular metabolism plays a crucial role in neuronal health, and dysregulation in metabolic pathways may contribute to HD pathogenesis.
Mechanisms of Action of Promising Drug Candidates
Drug candidates targeting these new targets employ diverse mechanisms of action. For example, anti-inflammatory drugs can modulate the activity of cytokines, thereby reducing neuroinflammation. Antioxidants can neutralize ROS, mitigating oxidative stress. Metabolic modulators can restore cellular metabolic balance, potentially promoting neuronal survival. The mechanisms of action of these compounds are often multifaceted, and understanding their intricate interactions is crucial for optimizing efficacy and safety.
Comparison and Contrast of Different Approaches
Different approaches to inhibiting or modulating these targets vary in their specific mechanisms and potential side effects. Anti-inflammatory drugs, for example, can be broadly categorized as non-steroidal anti-inflammatory drugs (NSAIDs) or corticosteroids, each with distinct pharmacological profiles and potential side effects. Antioxidant therapies can encompass various molecules, each with varying efficacy and potential interactions with other cellular processes.
Metabolic modulators are still a relatively new field, with a wide range of potential targets and mechanisms, requiring more research. Comparative studies are essential to identify the most effective and safest approaches.
Potential Benefits and Risks
Targeting these new mechanisms offers the potential for significantly improved outcomes in HD. Reducing neuroinflammation may alleviate neurodegenerative processes and improve motor and cognitive functions. Mitigation of oxidative stress can protect neurons from damage, preserving their function. Correcting metabolic imbalances could potentially enhance neuronal resilience. However, potential risks associated with these approaches need careful consideration.
For instance, anti-inflammatory drugs might have unwanted effects on other inflammatory processes in the body. Antioxidant therapies may not always be effective or may have unintended consequences. Metabolic modulators could have unforeseen interactions with other cellular pathways. Careful preclinical and clinical studies are essential to evaluate both the benefits and risks of each approach.
Molecular Pathways Involved in Huntington’s Disease
| Molecular Pathway | Potential Drug Targets | Mechanism of Action (Example) |
|---|---|---|
| Neuroinflammation | Cytokines (TNF-α, IL-1β), Inflammatory mediators | Inhibit cytokine production or activity, reducing neuroinflammation. |
| Oxidative Stress | Reactive Oxygen Species (ROS), Antioxidant enzymes | Increase antioxidant capacity, neutralizing ROS. |
| Cellular Metabolism | Key enzymes in glycolysis, mitochondrial function | Restore metabolic balance, improving neuronal energy production. |
| Mutant Huntingtin Aggregation | Protein chaperones, aggregation inhibitors | Prevent or reduce mutant huntingtin aggregation. |
Note: This table provides a simplified overview. Numerous other pathways and interactions are involved in Huntington’s Disease.
Clinical Trial Design and Methodology
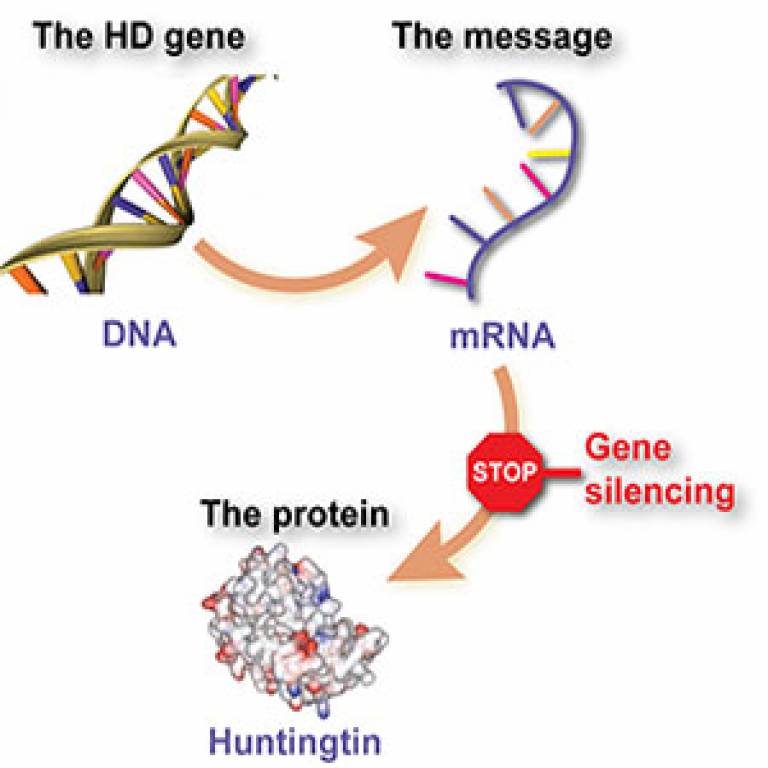
Bringing a new drug to treat Huntington’s disease requires meticulous planning and execution of clinical trials. These trials are crucial to demonstrate the drug’s safety and efficacy, ultimately paving the way for its potential approval and use by patients. Understanding the phases, protocols, and ethical considerations is essential for navigating the complexities of this process.
Phases of Clinical Trials
Clinical trials for Huntington’s disease drugs typically progress through several phases, each with distinct objectives. Phase 1 trials primarily focus on determining the drug’s safety profile in a small group of healthy volunteers or patients with the disease. Phase 2 trials expand the patient pool to evaluate the drug’s potential effectiveness and further refine its safety profile. Phase 3 trials, involving a larger number of patients, compare the new drug to existing treatments or a placebo, rigorously assessing its efficacy and long-term safety.
Phase 4 trials, often conducted after market approval, continue to monitor the drug’s safety and effectiveness in a broader patient population over time.
Clinical Trial Protocol Elements
A well-structured clinical trial protocol is essential for ensuring the integrity and validity of the study. Key elements include clearly defined inclusion and exclusion criteria to select participants who are most likely to benefit from the study and minimize potential biases. These criteria specify the characteristics, such as age, disease severity, and other medical conditions, that participants must meet to be enrolled.
Specific outcome measures, quantifiable metrics to assess the drug’s impact, are crucial for evaluating the treatment’s efficacy. These measures can include changes in motor function, cognitive performance, and disease progression. Robust safety monitoring is paramount, with regular assessments of adverse events and serious side effects. Data collection and analysis protocols ensure the integrity of the data gathered.
Examples of Trial Designs
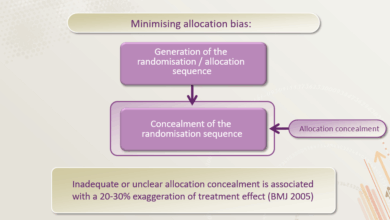
Various trial designs are employed in evaluating Huntington’s disease drugs. Randomized controlled trials (RCTs) are commonly used, assigning participants randomly to either the treatment group or a control group (receiving a placebo or standard therapy). This helps to minimize bias and assess the drug’s effect in a more objective manner. Double-blind studies, where neither the participants nor the researchers know who is receiving the treatment or placebo, further enhance the objectivity of the results.
Other designs, such as crossover trials, may be used in specific situations to assess the drug’s efficacy and safety over time.
Ethical Considerations and Regulatory Requirements
Ethical considerations are paramount in clinical trials. Informed consent from participants is essential, ensuring they understand the risks and benefits of participating. Data privacy and confidentiality must be rigorously maintained, protecting the personal information of trial participants. Regulatory bodies, like the FDA in the US, oversee clinical trials to ensure they adhere to strict standards of safety and ethical conduct.
Ethical review boards (IRBs) scrutinize the protocols to ensure ethical considerations are met. The rigorous regulatory process guarantees the safety and efficacy of the drug before it’s made available to patients.
Endpoints Used in Huntington’s Disease Drug Evaluation, New drug for huntingtons disease
| Endpoint Category | Description | Example Measures |
|---|---|---|
| Motor Function | Assessment of movement-related symptoms. | UPDRS motor score, timed tasks (e.g., finger tapping), gait analysis |
| Cognitive Function | Evaluation of cognitive abilities. | MMSE (Mini-Mental State Examination), neuropsychological tests (e.g., memory, attention), functional capacity questionnaires |
| Psychiatric Symptoms | Assessment of mood, anxiety, and behavioral issues. | HAM-D (Hamilton Depression Rating Scale), YMRS (Young Mania Rating Scale), behavioral rating scales |
| Disease Progression | Measurement of disease progression and symptom severity. | Unified Huntington’s Disease Rating Scale (UHDRS), disease duration, CAG repeat length |
| Safety | Monitoring for adverse events and side effects. | Frequency, severity, and duration of adverse events; vital signs, laboratory tests |
Potential Benefits and Risks of New Treatments
The quest for effective Huntington’s Disease (HD) treatments hinges on a careful evaluation of potential benefits and associated risks. This crucial assessment informs clinical trial design and guides the path toward improved symptom management and disease modification. Understanding the trade-offs is essential for patients and researchers alike, ensuring that potential therapies are both safe and impactful.The potential benefits of new HD treatments are multifaceted, encompassing symptom alleviation and, ideally, disease-modifying effects.
Successful therapies could significantly improve the quality of life for individuals living with HD by mitigating motor dysfunction, cognitive decline, and psychiatric disturbances. Furthermore, treatments aiming to slow or halt disease progression offer the prospect of extending lifespan and preserving functional abilities for longer periods. However, the pursuit of these benefits necessitates careful consideration of potential side effects and risks.
Potential Benefits of New HD Treatments
The potential benefits of new treatments extend beyond mere symptom management. Effective therapies could delay the onset of debilitating symptoms, thereby allowing patients more time to enjoy their lives and participate in meaningful activities. Further, some treatments may directly target the underlying causes of the disease, offering the potential for disease-modifying effects, thereby potentially halting or slowing the progression of HD.
Exciting news on a potential new drug for Huntington’s disease is certainly welcome, but it’s important to remember the devastating impact of events like the recent tragedy at Las Vegas hospitals. The outpouring of support for the victims and their families following the las vegas hospitals mass shooting highlights the need for compassion and resilience in the face of such horrific events.
Ultimately, continued research and development into treatments for Huntington’s disease remain crucial, and we hope for positive breakthroughs in the near future.
These benefits could significantly impact patients’ lives, offering improved quality of life and preserving functional abilities for a more extended period.
Potential Side Effects and Risks
New HD treatments, while promising, carry inherent risks and potential side effects. These could range from mild, transient reactions to more serious, long-lasting consequences. The severity and frequency of side effects can vary depending on the specific treatment and individual patient factors. Rigorous safety testing and close monitoring during clinical trials are essential to identify and mitigate these risks.
Understanding and managing these potential side effects is crucial for responsible treatment development and implementation.
Exciting news about a potential new drug for Huntington’s disease has me buzzing! It’s incredible to see progress in this area, and it’s inspiring to think about the potential impact on patients’ lives. However, the recent spotlight on HIV testing, like the ongoing increase in testing prompted by Charlie Sheen’s announcement ( charlie sheens hiv announcement still driving an increase in testing ), reminds us that proactive health awareness is crucial for all diseases, not just those with treatments in development.
Hopefully, the new drug for Huntington’s will be a game changer, and more advancements will follow!
Importance of Rigorous Safety Testing and Monitoring
Ensuring the safety of new HD treatments necessitates meticulous safety testing and ongoing monitoring during clinical trials. These trials should encompass a wide range of parameters, evaluating both short-term and long-term effects on various organ systems. Data collection should include detailed assessments of potential side effects, adverse reactions, and any unexpected outcomes. This proactive approach aims to minimize risks and maximize the probability of positive outcomes while minimizing harm to participants.
Risk-Benefit Assessments for New HD Treatments
Risk-benefit assessments for new HD treatments are complex, integrating a multitude of factors. These assessments consider the potential benefits in terms of symptom reduction and disease modification against the possible risks of side effects and long-term consequences. Expert panels and regulatory bodies weigh the data from clinical trials, considering factors like the severity of the disease, patient characteristics, and the potential impact on quality of life.
Ethical considerations play a critical role in these assessments, ensuring that the potential benefits outweigh the inherent risks.
Comparison of Potential Benefits and Risks of Different Drug Classes
| Drug Class | Potential Benefits | Potential Risks |
|---|---|---|
| Small molecule inhibitors of mutant huntingtin protein aggregation | May reduce the toxic effects of mutant huntingtin protein, potentially slowing disease progression. | Possible off-target effects on normal cellular processes, leading to unexpected side effects. |
| Neuroprotective agents | May promote neuronal survival and function, potentially reducing neuronal loss. | Risk of adverse effects on other brain functions or interactions with other medications. |
| Gene silencing therapies | Potentially alter the expression of the huntingtin gene, thereby reducing mutant huntingtin production. | Potential for unintended consequences on gene regulation and cellular function. |
| Immunotherapies | May target and eliminate abnormal immune responses contributing to HD pathology. | Risk of autoimmune reactions and potential inflammatory responses. |
Future Directions and Research Priorities: New Drug For Huntingtons Disease
The journey to conquering Huntington’s Disease (HD) demands a multifaceted approach. While current research has yielded valuable insights into the disease’s mechanisms and potential therapeutic targets, substantial challenges remain. Future research must address these challenges by exploring novel treatment strategies, fostering interdisciplinary collaborations, and embracing personalized medicine. This necessitates a commitment to public awareness and robust funding to ensure progress continues at a sustainable pace.
Promising Avenues for Future Research
Developing novel therapeutic strategies remains a critical area of focus. This involves exploring entirely new mechanisms of action, moving beyond current symptomatic treatments and aiming for disease-modifying therapies. Researchers are investigating gene silencing techniques, such as CRISPR-Cas9 gene editing, with the potential to directly target the mutated huntingtin gene. Also, repurposing existing drugs with potential off-target effects on HD pathways is another avenue under exploration.
These investigations hold the promise of altering the disease’s progression.
The Role of Interdisciplinary Collaborations
Interdisciplinary collaborations are essential for advancing research in HD. Bringing together experts from neuroscience, genetics, pharmacology, and computational biology creates a powerful synergy. This allows for a more comprehensive understanding of the disease’s complex pathophysiology and the development of more effective therapeutic strategies. For instance, neurologists can provide insights into the clinical presentation of HD, while geneticists can identify key genetic pathways involved in disease development.
Pharmacologists can then design and test new drug candidates. These collaborations foster the exchange of knowledge and expertise, ultimately accelerating progress.
Personalized Medicine Approaches
Personalized medicine approaches are poised to revolutionize HD treatment. The development of diagnostic tools capable of identifying individuals at risk or in the early stages of the disease can pave the way for early intervention. This will allow for tailored treatment plans based on individual genetic profiles and disease characteristics. For example, patients with specific genetic mutations might respond better to particular therapies.
By incorporating individual patient characteristics into treatment decisions, personalized medicine can maximize treatment efficacy and minimize side effects.
Public Awareness and Support
Public awareness and support are crucial for the success of HD research. Raising public awareness about the disease’s impact, symptoms, and current research initiatives can encourage participation in clinical trials and funding campaigns. This public engagement fosters a supportive environment for researchers and patients alike. Furthermore, raising public funds is vital for continuing critical research initiatives.
Current Research Funding Priorities
Current funding priorities in HD research focus on several key areas. These include funding clinical trials to assess the efficacy and safety of novel treatments, supporting basic research to better understand disease mechanisms, and investing in infrastructure and resources for researchers. Organizations like the Huntington’s Disease Society of America and other philanthropic groups are pivotal in allocating funds towards these essential research areas.
This allocation ensures that researchers have the resources to explore innovative strategies and contribute to the ultimate goal of finding a cure.
Illustrative Case Studies (Illustrative examples, not requiring detailed analysis)
This section presents hypothetical case studies to illustrate the diverse aspects of Huntington’s Disease (HD) and its potential treatments. These examples highlight the challenges and potential benefits associated with various approaches to managing the condition. Each case serves as a simplified illustration of the complexity of HD, avoiding exhaustive detail.
Hypothetical Patient Case Study: Initial Diagnosis and Treatment Options
A 45-year-old patient, initially presenting with subtle, yet increasing, motor difficulties, mood swings, and cognitive impairments, is diagnosed with Huntington’s Disease. The patient experiences difficulties with coordination, involuntary movements (chorea), and a progressive decline in cognitive function, affecting their ability to perform daily tasks. Treatment options include medication to manage chorea, counseling for emotional support, and therapies to address cognitive and physical impairments.
Impact of a New Drug Candidate on Symptom Management
A 32-year-old patient with Huntington’s Disease, experiencing moderate chorea and cognitive decline, participates in a clinical trial for a novel drug candidate. The drug, designed to target specific neurodegenerative pathways associated with HD, demonstrates a positive impact on chorea, reducing the frequency and severity of involuntary movements. The patient experiences an improvement in their ability to perform daily tasks, while maintaining a relatively stable cognitive function.
Efficacy of a Novel Treatment Strategy
A 58-year-old patient with advanced Huntington’s Disease, characterized by significant motor impairment and severe cognitive decline, participates in a clinical trial for a novel gene therapy treatment. The gene therapy aims to slow the progression of the disease by delivering therapeutic genetic material to modify disease-related processes within the nervous system. Preliminary results suggest a slower rate of cognitive and motor decline compared to the historical average.
Potential Side Effects of a New Drug
A 40-year-old patient with early-stage Huntington’s Disease, participating in a clinical trial for a new drug aimed at slowing the progression of the disease, experiences mild nausea and gastrointestinal discomfort as side effects. The patient reports that these side effects are manageable and do not significantly impact their quality of life. However, the drug’s efficacy is being closely monitored in the trial, weighing the benefits against the potential risks.
Patient Response to Combination Therapy
A 62-year-old patient with moderate Huntington’s Disease, exhibiting a combination of motor and cognitive symptoms, is treated with a combination therapy involving a new drug targeting specific neurodegenerative pathways along with physical therapy and cognitive rehabilitation. The patient demonstrates a noticeable improvement in both motor skills and cognitive function, highlighting the potential benefits of a multi-faceted approach to treatment.
Last Word
In conclusion, the quest for a new drug for Huntington’s disease presents a significant challenge, yet also a remarkable opportunity for progress. While significant hurdles remain, the ongoing research, clinical trials, and interdisciplinary collaborations offer hope for a brighter future for those affected by this debilitating condition. The potential benefits, coupled with a meticulous approach to risk assessment and safety monitoring, are crucial elements in moving forward.
This discussion underscores the vital need for continued research and support to effectively address the needs of individuals living with Huntington’s disease.