What do randomization and blinding mean in clinical trials? This exploration dives deep into the critical roles these methods play in ensuring reliable and unbiased results. Clinical trials are essential for advancing medical knowledge and improving patient care, but the meticulous design of these studies is crucial for trustworthy conclusions. Understanding the intricacies of randomization and blinding is key to grasping the entire process.
We’ll begin by examining the fundamental purpose of clinical trials, their historical context, and the different phases they encompass. Then, we’ll break down randomization, exploring its various types and how it minimizes bias. Following that, we’ll analyze the concept of blinding, discussing its different forms and the crucial role it plays in controlling for the placebo effect and other influences.
The discussion will also cover the practical applications of these techniques, along with the potential pitfalls and ethical considerations.
Introduction to Clinical Trials: What Do Randomization And Blinding Mean In Clinical Trials
Clinical trials are carefully designed experiments that evaluate the effectiveness and safety of new medical treatments, interventions, or diagnostic methods. They play a crucial role in advancing medical knowledge and improving patient outcomes. These trials involve human participants, and rigorous methodologies are employed to ensure reliable results. The ultimate goal is to determine whether a new therapy or procedure is safe, effective, and better than existing options.Clinical trials are essential for moving promising treatments from the laboratory to the clinic, ultimately benefiting patients.
Randomization and blinding are integral components of this process, ensuring that results are not skewed by bias and that treatments are fairly compared. The historical evolution of clinical trial design reflects the growing understanding of the importance of rigorous scientific methodology in evaluating medical interventions.
Randomization and blinding are crucial elements in clinical trials, ensuring unbiased results. They help researchers determine if a treatment is truly effective, not just due to other factors. For example, in rheumatoid arthritis clinical trials, rheumatoid arthritis clinical trials often use these methods to fairly compare different therapies. Ultimately, randomization and blinding help researchers avoid bias and draw accurate conclusions about the treatment’s impact.
The Fundamental Need for Randomization and Blinding
Randomization ensures that participants are assigned to different treatment groups (e.g., experimental drug vs. placebo) by chance, minimizing selection bias. This approach prevents researchers from consciously or unconsciously influencing participant allocation. Blinding, either single- or double-blind, conceals knowledge of treatment assignment from either the participant or both the participant and the researcher. This helps eliminate bias in data collection and interpretation.
By concealing who is receiving which treatment, it prevents the expectation of a particular outcome from influencing the results. For instance, if a patient knows they are receiving a new drug, their expectations could affect their response, potentially masking the drug’s true effectiveness.
Historical Context of Clinical Trial Methodologies
The evolution of clinical trial methodologies reflects a growing understanding of the need for rigorous scientific approaches to evaluating medical interventions. Early trials often lacked the standardized procedures and controls that are now considered essential. The development of the randomized controlled trial (RCT) in the mid-20th century was a pivotal moment, marking a shift towards more objective and unbiased assessments of treatment efficacy.
This change stemmed from a recognition of the potential for bias in observational studies and the need for a more systematic approach to research. The Belmont Report, published in the 1970s, further shaped clinical trial ethics and emphasized the importance of informed consent and participant safety.
Phases of Clinical Trials
Understanding the different phases of clinical trials provides insight into the progression of a new treatment from initial testing to potential widespread use. Each phase has specific goals and characteristics.
| Phase | Description | Goal | Number of Participants |
|---|---|---|---|
| Phase 1 | Evaluates safety, tolerability, and optimal dosage of a new treatment. | Determine the maximum tolerated dose (MTD) and identify potential side effects. | Usually 20-80 |
| Phase 2 | Assesses the treatment’s effectiveness in a larger group of patients. | Assess efficacy and further evaluate side effects. | Usually 100-300 |
| Phase 3 | Compares the new treatment to a standard treatment (or placebo) in a large, diverse patient population. | Confirm effectiveness, monitor side effects, and compare the new treatment to existing options. | Usually 300-3000+ |
| Phase 4 | Monitors the long-term effects and safety of the treatment after it has been approved for use. | Collect data on long-term side effects, effectiveness in diverse populations, and identify optimal use strategies. | Thousands |
Randomization
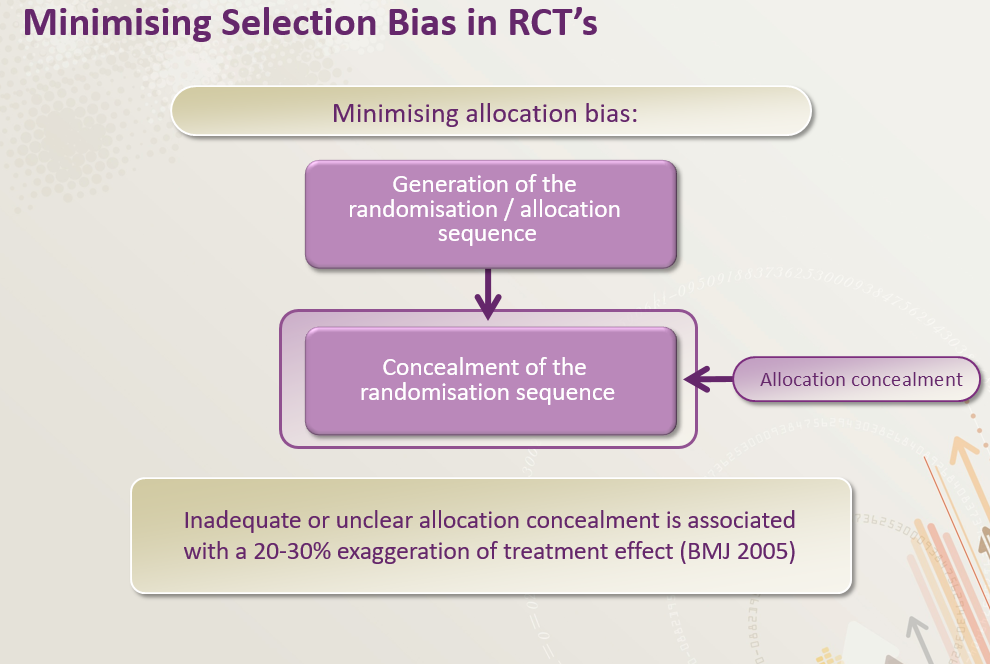
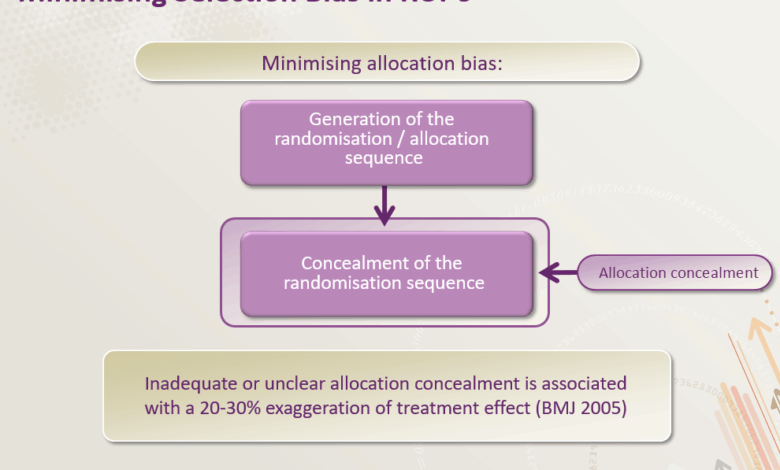
Randomization is a cornerstone of clinical trials, ensuring that participants are assigned to different treatment groups in a fair and unbiased way. This process helps to minimize the influence of confounding factors and ensures that any observed differences in outcomes are truly due to the treatments being compared, not to pre-existing differences between the groups. It’s a crucial tool for maintaining the integrity and validity of the trial results.Randomization effectively removes the potential for researchers or participants to influence the allocation of treatments, creating a more objective comparison.
By using a random process, researchers can eliminate conscious or unconscious biases that might otherwise skew the results. This crucial step is vital in establishing a cause-and-effect relationship between the intervention and the observed outcomes.
Types of Randomization Methods
Randomization methods are designed to produce comparable groups. Different methods offer varying degrees of control and are appropriate for different trial designs. Understanding these methods is essential for evaluating the validity of a clinical trial.
Randomization and blinding are crucial in clinical trials to ensure unbiased results. Basically, they aim to minimize the influence of outside factors on the outcome. For example, in research exploring leafy greens multiple sclerosis treatment leafy greens multiple sclerosis treatment , randomly assigning participants to different groups and keeping them blind to which group they’re in helps scientists pinpoint the actual effect of leafy greens on MS.
This way, they can better determine if leafy greens are truly helpful, or if other variables are playing a role. Without proper randomization and blinding, it’s hard to draw accurate conclusions from the study.
- Simple Randomization: This method assigns participants to groups using a random process, like a coin flip or a random number generator, with each participant having an equal chance of being assigned to any group. It’s straightforward to implement but can lead to imbalances in the characteristics of the groups if the sample size is small.
- Stratified Randomization: This method addresses the potential for imbalances in baseline characteristics. Researchers divide participants into strata (subgroups) based on important factors (e.g., age, gender, disease severity). Then, simple randomization is applied within each stratum, ensuring that the groups have comparable characteristics within each subgroup. This approach is more complex but can produce more balanced groups, especially in trials with smaller sample sizes.
- Block Randomization: This method involves creating blocks of participants of a specific size (e.g., 4). Within each block, participants are randomly assigned to treatment groups in a balanced manner. This ensures that the proportion of participants in each group remains relatively constant throughout the trial, reducing the risk of imbalances between treatment groups. This method is particularly helpful when dealing with limited participant availability or ensuring balance between treatment arms in a trial.
Examples of Randomization in Clinical Trials
Randomization is widely applied across various clinical trial scenarios. For example, in a trial comparing a new drug to a standard treatment for high blood pressure, participants would be randomly assigned to either the new drug or the standard treatment group. This ensures that potential differences in the effectiveness of the two treatments are not due to pre-existing differences between the participants in the groups.
Another example could be a study evaluating the effectiveness of different surgical techniques for treating a specific type of cancer. Randomizing patients to different surgical procedures ensures that any observed differences in outcomes are due to the procedures themselves, not to other factors that may influence the patients’ health.
Importance of Random Number Generation
The use of random number generation is essential for the validity and integrity of clinical trials. Random number generators produce sequences of numbers that have no predictable pattern. This ensures that each participant has an equal chance of being assigned to any treatment group, eliminating bias in the allocation process.
Randomization and blinding are crucial in clinical trials to ensure unbiased results. Basically, they help prevent researchers from influencing the outcome. For example, in the bigfoot unity connected diabetes system , understanding how these methods are applied is essential to the accuracy of the results, and therefore, how effective the system is. This is because these principles help avoid bias in evaluating the impact of the system on patients’ health.
Ultimately, proper randomization and blinding are key to reliable conclusions in any clinical trial.
Random number generation is a crucial aspect of randomization, ensuring unbiased allocation to treatment groups.
Comparing Randomization Methods
| Method | Description | Advantages | Disadvantages |
|---|---|---|---|
| Simple Randomization | Participants randomly assigned to groups. | Easy to implement. | Can lead to imbalances in baseline characteristics, especially with small sample sizes. |
| Stratified Randomization | Randomization within subgroups based on characteristics. | Reduces imbalances in baseline characteristics. | More complex to implement. |
| Block Randomization | Randomization within blocks of a fixed size. | Maintains balance between groups. | Requires careful planning to ensure block size is appropriate. |
Blinding (Masking)
Blinding, also known as masking, is a crucial technique in clinical trials that aims to minimize bias in study results. By concealing information about treatment assignments from those involved in the trial, blinding helps ensure that the outcome is primarily determined by the treatment itself, rather than by expectations or other external factors. This critical step significantly enhances the reliability and validity of the trial findings.Blinding prevents various biases, including those related to the participants’ expectations, the researchers’ interpretations, and the assessors’ evaluations.
By removing these potential sources of bias, blinding allows for a more accurate assessment of the treatment’s true effectiveness and safety. This ultimately contributes to more reliable and trustworthy medical advancements.
Types of Blinding
Blinding strategies come in various forms, each with different levels of concealment. Understanding these variations is key to appreciating the strengths and limitations of each approach.
- Single-blind: In this type of blinding, only one group—either the participants or the study personnel—is unaware of the treatment assignments. For example, participants may not know if they are receiving the experimental drug or a placebo, while researchers administering the treatment know the assignment. This reduces the bias of participants’ expectations.
- Double-blind: In a double-blind trial, neither the participants nor the study personnel involved in assessing outcomes are aware of the treatment assignments. This is achieved by using coded treatment assignments and independent personnel to collect and analyze data. This approach minimizes both participant expectation bias and researcher bias.
- Triple-blind: Taking blinding a step further, a triple-blind trial adds another layer of concealment. In this design, not only are the participants and assessing personnel unaware of treatment assignments, but also the data analysts analyzing the results are blinded. This stringent level of blinding minimizes the potential for any bias to influence the analysis and interpretation of the trial data.
Real-World Examples
Blinding is routinely used in various clinical trial designs. For instance, in a study evaluating a new blood pressure medication, patients and the nurses recording blood pressure readings might be blinded to the treatment assignment. This ensures that the nurses’ assessments are not influenced by knowing whether a patient is taking the new drug or a placebo.Another example involves a clinical trial investigating the efficacy of a new pain reliever.
Researchers might use a double-blind design, where neither the patients nor the doctors assessing pain levels know which patients are receiving the new medication and which are receiving a placebo. This helps to eliminate bias in both groups.
Controlling for Bias and Improving Validity
Blinding effectively controls for various biases. By masking the treatment assignments, blinding reduces the influence of participant expectations and researcher/personnel subjectivity. This leads to more reliable and valid results, increasing the likelihood that the observed effects are directly attributable to the treatment intervention rather than other factors.
Challenges and Limitations
Despite its advantages, blinding presents challenges in specific situations. For example, in trials involving surgery, blinding the surgeon is often not possible or ethical. The surgical technique itself may be a confounding factor.Similarly, in trials assessing psychological interventions, blinding participants or assessors can be difficult or impossible. The patient’s subjective experience, and the assessor’s interpretation of that experience, can be influenced by factors beyond the treatment.
Furthermore, blinding is often more challenging in observational studies, where researchers cannot directly manipulate the exposure.
Randomization and Blinding in Practice
Randomization and blinding are crucial components of high-quality clinical trials. They help researchers minimize bias and ensure that any observed differences in outcomes are truly due to the treatment being tested, rather than other factors. This section delves into the practical application of these techniques, examining their use in various disease areas, potential pitfalls, and mitigation strategies.Randomization and blinding are employed in tandem to minimize the influence of confounding variables.
Random assignment ensures that the characteristics of the treatment and control groups are similar, making it easier to isolate the effects of the treatment. Blinding, by masking participants and often researchers, prevents subjective assessments from skewing the results.
Using Randomization and Blinding to Control Confounding Variables
Randomization helps control for known and unknown confounding variables by distributing these variables equally across the treatment and control groups. This equal distribution reduces the likelihood that pre-existing differences between the groups will influence the results. For instance, if age is a potential confounding variable, randomization ensures that the average age is roughly the same in both groups.
Blinding further strengthens this control by eliminating the possibility of biased assessments. A physician prescribing a treatment, knowing which group the patient belongs to, might unconsciously influence the patient’s care, potentially skewing results.
Potential Pitfalls in Applying Randomization and Blinding
While randomization and blinding are powerful tools, there are potential pitfalls to consider. One pitfall is non-compliance with the randomization schedule. If participants or researchers deviate from the assigned treatment or protocol, the randomization process loses its strength. This can be mitigated by careful monitoring and strict adherence to the protocol. Another pitfall is inadequate blinding.
If the blinding is not thorough enough, subtle cues might reveal the treatment assignment, influencing the results. A researcher might subtly adjust the dosage or treatment schedule based on the perceived group, thus compromising the study.
Randomization and Blinding in Specific Disease Areas
Randomization and blinding are employed across various disease areas. In oncology, trials often use randomization to compare different chemotherapy regimens or targeted therapies. Blinding is crucial to prevent bias in assessing tumor response or side effects. In cardiology, randomization helps compare different medications or procedures for treating heart conditions. Blinding is critical in assessing symptoms, blood pressure, and other physiological responses, preventing bias in the interpretation of results.
Randomization and Blinding Across Different Types of Clinical Trials
| Trial Type | Randomization | Blinding |
|---|---|---|
| Phase I Trials | Often not randomized, focused on safety and dose | Generally not blinded |
| Phase II Trials | May or may not be randomized, exploring efficacy | May be blinded |
| Phase III Trials | Almost always randomized, comparing efficacy of treatments | Often blinded, especially for efficacy outcomes |
| Phase IV Trials | Usually randomized, evaluating long-term effects and safety | Often blinded |
Ethical Considerations in Randomization and Blinding Procedures
Ethical considerations are paramount in clinical trials. Randomization must be implemented fairly, ensuring all participants have an equal chance of receiving any treatment. Blinding procedures must not compromise patient safety or access to essential information. For example, if a treatment is known to have severe side effects, unblinding might be necessary to ensure patient well-being. These procedures should be reviewed and approved by independent ethical review boards to protect patient rights and interests.
Impact of Randomization and Blinding
Proper randomization and blinding are crucial for ensuring the validity and reliability of clinical trial results. These techniques are designed to minimize bias, enhance the generalizability of findings, and improve the statistical power of the analyses. By creating a more controlled environment, researchers can gain a clearer understanding of the true effect of the treatment being studied.
Effect on Validity and Reliability
Randomization and blinding directly impact the validity and reliability of clinical trial results by minimizing bias. Random assignment ensures that the treatment groups are comparable at the start of the study, reducing the likelihood that pre-existing differences between participants influence the outcomes. Blinding prevents subjective assessments from influencing the results, ensuring that both participants and researchers are unaware of the treatment assignment.
This objectivity leads to more accurate and trustworthy conclusions.
Improving Generalizability
Randomization and blinding increase the generalizability of trial findings by creating a more representative sample of the target population. A well-randomized sample is more likely to reflect the characteristics of the broader population, allowing researchers to extrapolate the results to a wider range of individuals. This ensures that the study’s findings are not limited to a specific group, but rather applicable to a larger population.
Examples of Flawed Procedures
A trial investigating a new blood pressure medication might suffer from flawed randomization if participants with significantly higher baseline blood pressure were disproportionately assigned to the treatment group. This could lead to an overestimation of the medication’s effectiveness, as the observed improvement might be partly due to the pre-existing higher blood pressure levels. Similarly, if the researchers assessing the treatment’s efficacy were aware of the treatment assignment, their subjective assessments might inadvertently skew the results.
This highlights the critical importance of rigorous randomization and blinding procedures.
Impact on Statistical Analysis
Randomization allows researchers to use statistical methods appropriate for comparing groups. These methods, like t-tests or ANOVA, assume that the groups are independent and randomly selected. Blinding helps maintain objectivity in the data collection and analysis phase, reducing the risk of bias. For example, a study measuring patient pain levels might have different results if researchers evaluating the pain reports were aware of the treatment group assignments.
Blinding helps eliminate this potential bias. Statistical significance of the results is enhanced when the randomization and blinding procedures are appropriately implemented.
Consequences of Improper Implementation, What do randomization and blinding mean in clinical trials
Failure to implement proper randomization and blinding procedures can lead to several negative consequences. Biased results might incorrectly demonstrate the effectiveness or harmfulness of a treatment, potentially leading to incorrect clinical decisions. Such errors can lead to the development of ineffective treatments or the marketing of harmful products. This can waste resources, delay the development of effective treatments, and ultimately cause harm to patients.
The credibility of the entire research field can also be undermined. Furthermore, a lack of transparency in these procedures can create mistrust among stakeholders and the public. This will affect the acceptance and implementation of valid research.
Methods for Evaluating Randomization and Blinding
Evaluating the quality of randomization and blinding in clinical trials is crucial for ensuring the validity and reliability of the results. Properly implemented randomization and blinding minimize bias, allowing researchers to confidently attribute any observed effects to the treatment being studied. Robust evaluation methods provide confidence in the trial’s integrity and contribute to the generalizability of findings to the wider population.
Assessing the Quality of Randomization
Randomization procedures aim to allocate participants to treatment groups in a way that doesn’t favor either group. Several methods are used to assess the quality of randomization. These include examining the balance of baseline characteristics between treatment groups, a critical indicator of unbiased allocation.
- Baseline Characteristic Balance: Analyzing the distribution of key variables (age, gender, disease severity) across treatment arms is fundamental. Significant imbalances might suggest flaws in the randomization process. For instance, if one group has a disproportionately higher number of patients with a specific pre-existing condition, it raises concerns about the randomization’s effectiveness.
- Statistical Tests for Randomness: Statistical tests, such as the chi-squared test or the run test, can evaluate the randomness of the allocation sequence. These tests examine whether the observed distribution of participants across groups deviates significantly from what would be expected under a truly random allocation. A significant deviation suggests the randomization procedure may have introduced bias.
Assessing the Quality of Blinding
Blinding (masking) protects participants and researchers from knowing which treatment a participant received. This helps prevent subjective assessments from influencing the outcome. Evaluating the degree of blinding is crucial.
- Observer Blinding: Assessing whether researchers involved in data collection (e.g., nurses, physicians) were blinded is essential. This involves evaluating the design of the trial and how the treatment assignments were concealed from those observers. For example, if the treatment is administered in a distinctive manner, and the observers know the treatment assignment by its physical characteristics, this constitutes a significant lack of blinding.
- Participant Blinding: Measuring participant blinding involves understanding how the trial design ensured participants didn’t know their treatment assignment. This includes aspects like identical-looking pills or placebo treatments. A lack of participant blinding can introduce bias if participants adjust their behavior based on their perceived treatment.
Tools and Checklists for Assessing Randomization and Blinding
Various tools and checklists aid in evaluating the quality of randomization and blinding procedures. These tools provide a structured approach to assess the potential for bias in the trial.
| Tool/Checklist | Description |
|---|---|
| CONSORT Statement | The Consolidated Standards of Reporting Trials (CONSORT) statement provides a checklist for reporting clinical trials. Components of this statement specifically address randomization and blinding procedures, offering guidance on how to document these processes adequately. |
| Cochrane Handbook for Systematic Reviews of Interventions | This handbook offers detailed guidance on assessing the risk of bias in randomized controlled trials (RCTs). It provides a structured approach to evaluating the randomization and blinding procedures, allowing researchers to critically evaluate the study’s design. |
Criteria for Evaluating Randomization and Blinding Procedures
Evaluations of randomization and blinding procedures often utilize a set of criteria that focus on the extent to which the procedures were implemented appropriately. The criteria typically encompass the following:
- Adequacy of Randomization Method: The method used for randomization should be clearly defined and demonstrably random. This may include using a computer-generated sequence or a random number table.
- Concealment of Allocation: Procedures should be in place to conceal the treatment allocation from those involved in the trial, ensuring the randomization process isn’t influenced by anyone’s knowledge of the allocation sequence.
- Maintenance of Blinding: Strategies should be implemented to maintain blinding throughout the study. Examples include using identical-appearing placebo treatments or coding the treatment assignments.
Final Summary
In conclusion, randomization and blinding are indispensable components of robust clinical trials. Their proper implementation directly impacts the validity and reliability of results, enabling researchers to draw more accurate conclusions about the effectiveness of treatments. We’ve covered the critical importance of these methodologies, their various types, and the crucial role they play in generating trustworthy results. By understanding these methods, we gain a deeper appreciation for the rigor and precision inherent in the scientific pursuit of medical advancements.